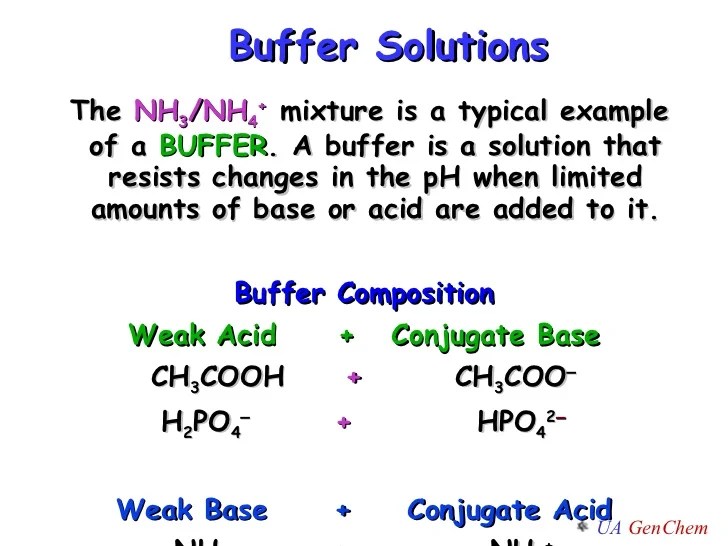
Buffer Compound Chemistry . Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes: Calculate the ph of a buffer before and after the addition of added acid or base. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. What is buffer in chemistry? Ions are atoms or molecules that have lost or.
from www.slideshare.net
Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Ions are atoms or molecules that have lost or. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from that weak acid. What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. It is able to neutralize small. Calculate the ph of a buffer before and after the addition of added acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
Lect w9 152 buffers and ksp_alg
Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of a buffer before and after the addition of added acid or base. Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. What is buffer in chemistry? It is able to neutralize small. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Ions are atoms or molecules that have lost or.
From loeqokhbo.blob.core.windows.net
Buffer Compound Example at Lloyd Ogrady blog Buffer Compound Chemistry Either a weak acid plus a salt derived from that weak acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Ions are atoms or molecules that have lost or. A buffer is a solution that can resist ph change upon the addition of. Buffer Compound Chemistry.
From www.peoi.org
Chapter 12 Section G Buffers Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. What is buffer in chemistry? Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism. Buffer Compound Chemistry.
From www.chegg.com
Solved Which set of compounds would form a buffer in aqueous Buffer Compound Chemistry Ions are atoms or molecules that have lost or. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution whose ph is not altered to any great extent by the addition of. Buffer Compound Chemistry.
From www.chegg.com
Solved Which pair of compounds will form a buffer in aqueous Buffer Compound Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Either a weak acid plus a salt derived from that weak acid. What is buffer in chemistry? The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being. Buffer Compound Chemistry.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at Buffer Compound Chemistry Either a weak acid plus a salt derived from that weak acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer, in chemistry, solution usually containing an acid and a. Buffer Compound Chemistry.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Buffer Compound Chemistry The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid. Calculate the ph of a buffer. Buffer Compound Chemistry.
From www.chegg.com
Solved Which pair of compounds will form a buffer in aqueous Buffer Compound Chemistry Calculate the ph of a buffer before and after the addition of added acid or base. Ions are atoms or molecules that have lost or. It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Buffer, in chemistry, solution. Buffer Compound Chemistry.
From www.slideserve.com
PPT Atoms, Molecules, Ions, Acids, Bases, Buffers, pH PowerPoint Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Calculate the ph of a buffer before and after the addition of added acid or base. What is buffer in chemistry? Ions are atoms or molecules that have lost or. Buffers do so by being composed of. Buffer Compound Chemistry.
From www.chegg.com
Solved Which pair of compounds will form a buffer in aqueous Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Calculate the ph of a buffer before and after the addition of added acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is. Buffer Compound Chemistry.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Compound Chemistry A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Ions are atoms or molecules that have lost or. Calculate. Buffer Compound Chemistry.
From www.researchgate.net
Absorption of different buffer compounds and salts in the farUV a Buffer Compound Chemistry What is buffer in chemistry? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from that weak acid. Buffer, in chemistry, solution usually containing an acid and a base,. Buffer Compound Chemistry.
From www.dreamstime.com
Buffer Solution in Glass, Chemical in the Laboratory and Industry Stock Buffer Compound Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Calculate the ph of a buffer before and after the addition of added acid or base. What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt,. Buffer Compound Chemistry.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson Buffer Compound Chemistry Either a weak acid plus a salt derived from that weak acid. It is able to neutralize small. Ions are atoms or molecules that have lost or. Buffers do so by being composed of certain pairs of solutes: Calculate the ph of a buffer before and after the addition of added acid or base. A buffer is a solution that. Buffer Compound Chemistry.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. The mechanism involves a buffer, a solution that resists dramatic changes in ph. What is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of either. Buffer Compound Chemistry.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Buffer Compound Chemistry It is able to neutralize small. What is buffer in chemistry? Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. A buffer is a solution that can resist ph change upon the addition. Buffer Compound Chemistry.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Compound Chemistry Ions are atoms or molecules that have lost or. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of a buffer before and after the addition of added acid or base. The mechanism involves a buffer,. Buffer Compound Chemistry.
From www.chegg.com
Solved Which pair of compounds will form a buffer in aqueous Buffer Compound Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: What is buffer in chemistry? A buffer is a solution that. Buffer Compound Chemistry.
From study.com
Quiz & Worksheet The Buffer System in Chemistry Buffer Compound Chemistry The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities. Buffer Compound Chemistry.
From www.youtube.com
Which pair of compounds will form a buffer in an aqueous solution? a Buffer Compound Chemistry Either a weak acid plus a salt derived from that weak acid. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution whose ph is not altered to any great extent by. Buffer Compound Chemistry.
From www.alamy.com
Buffer reagent hires stock photography and images Alamy Buffer Compound Chemistry Calculate the ph of a buffer before and after the addition of added acid or base. Buffers do so by being composed of certain pairs of solutes: It is able to neutralize small. Either a weak acid plus a salt derived from that weak acid. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that. Buffer Compound Chemistry.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Ions are atoms or molecules that. Buffer Compound Chemistry.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1031156 Buffer Compound Chemistry Ions are atoms or molecules that have lost or. Either a weak acid plus a salt derived from that weak acid. What is buffer in chemistry? The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. Calculate the ph of a buffer before and after the addition of added acid or. Buffer Compound Chemistry.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Compound Chemistry Buffers do so by being composed of certain pairs of solutes: Calculate the ph of a buffer before and after the addition of added acid or base. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. A buffer is a solution that can resist. Buffer Compound Chemistry.
From chemicalformulaservices.com
Metal Buffing Bar Compound Formulation At 1 Chemical Formula Services Buffer Compound Chemistry Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. It is able to neutralize small. Calculate the ph of a buffer before and after the addition of added acid or base. The mechanism. Buffer Compound Chemistry.
From www.numerade.com
SOLVED The diagrams shown above contain one or more of the compounds Buffer Compound Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. It is able to neutralize small. Either a weak acid plus a salt derived from that weak acid. Calculate the ph of a buffer before and after the addition of added acid or base. Buffers do so. Buffer Compound Chemistry.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide Buffer Compound Chemistry The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. What is buffer in chemistry? Buffers do so by being composed of certain pairs of solutes:. Buffer Compound Chemistry.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Buffer Compound Chemistry Calculate the ph of a buffer before and after the addition of added acid or base. What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. It is able to neutralize small. A buffer is a solution that can resist ph change. Buffer Compound Chemistry.
From region-online-banking81.blogspot.com
What Is Meant By Buffer Solution Example Ella Scholten Coiffure Buffer Compound Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. It is able to neutralize small. Calculate the ph of a buffer before and after the addition of added acid or base. Ions are atoms or molecules that have lost or. Buffer, in chemistry, solution. Buffer Compound Chemistry.
From www.slideshare.net
Lect w9 152 buffers and ksp_alg Buffer Compound Chemistry Either a weak acid plus a salt derived from that weak acid. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Calculate the ph of a buffer before and after the addition of added acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. Buffer Compound Chemistry.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Buffer Compound Chemistry Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid. It is able to neutralize small. Calculate the ph of a buffer before and. Buffer Compound Chemistry.
From www.researchgate.net
Chemical structures of the pH buffers. MES, HEPES, and TAPS are Buffer Compound Chemistry Buffers do so by being composed of certain pairs of solutes: Calculate the ph of a buffer before and after the addition of added acid or base. It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. What is. Buffer Compound Chemistry.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Compound Chemistry A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends. Buffer Compound Chemistry.
From backxecalu.weebly.com
Chemistry Buffer Solutions Pdf !LINK! Buffer Compound Chemistry It is able to neutralize small. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution whose ph is not altered. Buffer Compound Chemistry.
From www.numerade.com
SOLVED Write ionic equations that illustrate how each pair of Buffer Compound Chemistry Ions are atoms or molecules that have lost or. It is able to neutralize small. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Calculate the. Buffer Compound Chemistry.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Buffer Compound Chemistry The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of a buffer before and after the addition of added acid or base. Buffers do so by being composed of certain pairs of solutes: A. Buffer Compound Chemistry.