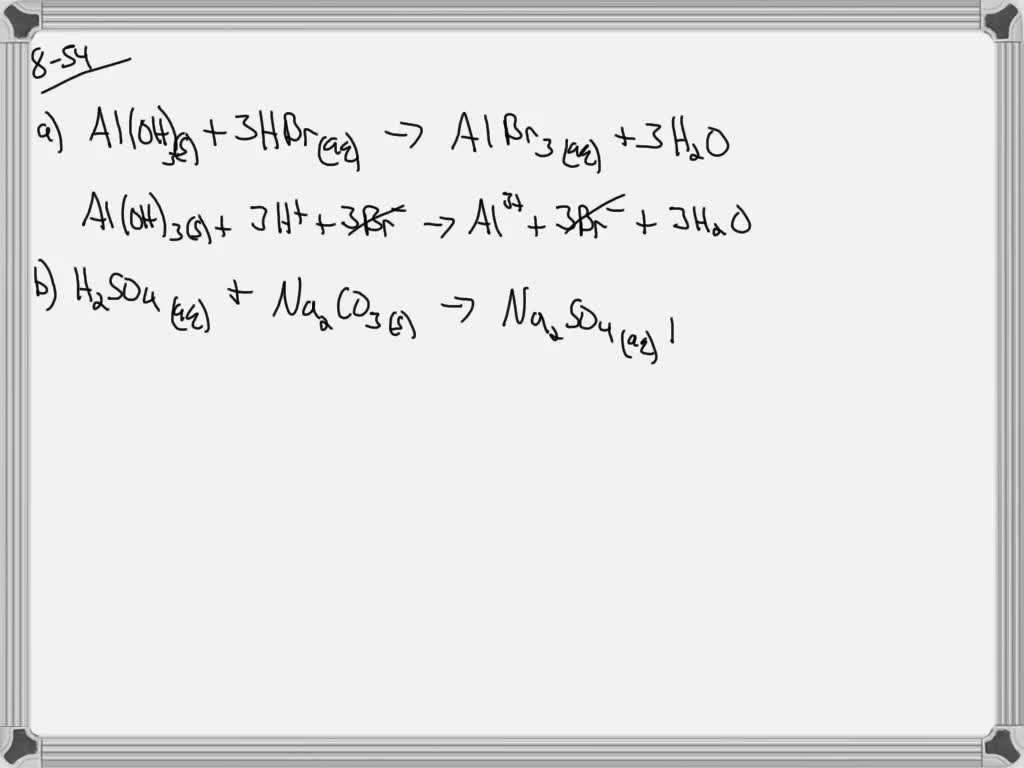Lead(Ii) Nitrate Reacts With Hydrochloric Acid . The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Fe + hcl = h2 + fecl2 To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). In a double replacement reaction, the cations. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate.
from www.numerade.com
In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Fe + hcl = h2 + fecl2 The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. In a double replacement reaction, the cations. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button.
SOLVEDand net ionic equation for the following eactions Write = balanced molecular insoluble
Lead(Ii) Nitrate Reacts With Hydrochloric Acid The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). In a double replacement reaction, the cations. Fe + hcl = h2 + fecl2 The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate.
From www.chegg.com
Solved 2. If 2.51 moles of lead (II) nitrate reacts with Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 In a double replacement reaction, the cations. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead (ii) chloride, a white precipitate, is formed. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Which type of chemical reaction take place when silver nitrate react with hydrochloric Lead(Ii) Nitrate Reacts With Hydrochloric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). In a double replacement reaction, the cations. The reaction between lead (ii) nitrate and hydrochloric. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.slideshare.net
Acids And Bases Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. The reaction between lead. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.chegg.com
When 174 mL of 0.870M lead(II) nitrate reacts with Lead(Ii) Nitrate Reacts With Hydrochloric Acid Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button.. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 In a double replacement reaction, the cations. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED 2.Aqueous solutions of aqueous lead(II) nitrate and sodium iodide are mixed. Show three Lead(Ii) Nitrate Reacts With Hydrochloric Acid In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.showme.com
ShowMe net ionic equation Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction,. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.coursehero.com
[Solved] Lead(II) nitrate reacts with potassium chromate to form lead(II)... Course Hero Lead(Ii) Nitrate Reacts With Hydrochloric Acid In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. The reaction between lead (ii) nitrate and hydrochloric acid is a. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Part I Observing Double Displacement Reactions Write the ionic formulas for the Lead(Ii) Nitrate Reacts With Hydrochloric Acid In a double replacement reaction, the cations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Identify the reactants and products in the given chemical equation, then check if any of the. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii) nitrate Precipitation Lead(Ii) Nitrate Reacts With Hydrochloric Acid In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Fe + hcl = h2 + fecl2 The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In a double replacement reaction,. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From klaalfdoh.blob.core.windows.net
Lead (Ii) Nitrate Reacts With Sodium Phosphate at James McCord blog Lead(Ii) Nitrate Reacts With Hydrochloric Acid The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In a double replacement reaction, the cations. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. In our balanced reaction, the stoichiometry reveals that 2 moles of. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Write balanced chemical equations. 2) Aqueous lithium hydroxide neutralizes sulfuric Lead(Ii) Nitrate Reacts With Hydrochloric Acid The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Fe + hcl = h2 + fecl2 To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In a double replacement reaction, the cations. Lead (ii) chloride, a white precipitate, is formed by. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Lead(Ii) Nitrate Reacts With Hydrochloric Acid In a double replacement reaction, the cations. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Fe + hcl = h2 + fecl2 The reaction between. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced net ionic equation for the reaction of aqueous solutions of lead(II Lead(Ii) Nitrate Reacts With Hydrochloric Acid Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Fe + hcl = h2 + fecl2 To balance a chemical. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.chegg.com
Solved The precipitation reaction of lead(II) nitrate with Lead(Ii) Nitrate Reacts With Hydrochloric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Identify the reactants and products in the given chemical equation, then check if any of. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From kladflymy.blob.core.windows.net
Lead Nitrate Hydroxide Balanced Equation at Jennifer Robin blog Lead(Ii) Nitrate Reacts With Hydrochloric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Lead(Ii) Nitrate Reacts With Hydrochloric Acid In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Fe + hcl = h2 + fecl2 In a double replacement reaction, the cations. Identify the reactants and products in the given chemical equation, then check if any of the products form. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Zn(NO3)2 = NaNO3 + ZnS YouTube Lead(Ii) Nitrate Reacts With Hydrochloric Acid Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). To balance a chemical equation, enter an equation of a. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts to form solid lead(II Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. To balance a chemical equation, enter an equation of a chemical reaction. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Lead (II) oxide reacts with hydrochloric acid in a double displacement reaction. How Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In a double replacement reaction, the cations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead (ii) chloride, a white precipitate, is formed by. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From klaiketkj.blob.core.windows.net
Aluminum Chloride Reacts With Lead Ii Nitrate at Becky Warren blog Lead(Ii) Nitrate Reacts With Hydrochloric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In a double replacement reaction, the cations. Fe + hcl = h2 + fecl2 Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. The reaction between lead (ii) nitrate and hydrochloric. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double displacement reaction with Lead(Ii) Nitrate Reacts With Hydrochloric Acid In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In a double replacement reaction, the cations. Identify the reactants and products. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Solid nickel reacts with aqueous lead (II) nitrate to form solid lead. What is the net Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. To balance a. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid lead(II) sulfide reacts Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. In a double replacement reaction, the cations. In our balanced reaction, the stoichiometry reveals that. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.chegg.com
Solved Lead (II) nitrate reacts with sodium iodide to Lead(Ii) Nitrate Reacts With Hydrochloric Acid The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In a double replacement reaction, the cations. Fe + hcl = h2 + fecl2 To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In our balanced reaction, the stoichiometry reveals that 2. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.youtube.com
Lead Nitrate + Hydrochloric Acid YouTube Lead(Ii) Nitrate Reacts With Hydrochloric Acid In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Fe + hcl = h2 + fecl2 Identify the reactants and products in the given. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Texts What is the precipitate that forms when aqueous lead(II) nitrate reacts with Lead(Ii) Nitrate Reacts With Hydrochloric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In a double replacement reaction, the cations. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Lead (ii) chloride, a white precipitate, is formed. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write = balanced molecular insoluble Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 In a double replacement reaction, the cations. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the following balanced equation Lead(Ii) Nitrate Reacts With Hydrochloric Acid Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. In a double replacement reaction, the cations. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Fe + hcl = h2 + fecl2 To balance a chemical. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lead(Ii) Nitrate Reacts With Hydrochloric Acid In a double replacement reaction, the cations. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. Fe + hcl =. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.chegg.com
Solved 2) When lead (II) nitrate reacts with sodium iodide, Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In a double replacement reaction, the cations. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Lead (ii) chloride, a white precipitate, is formed by. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104 Lead(Ii) Nitrate Reacts With Hydrochloric Acid Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. In our balanced reaction, the stoichiometry reveals that 2 moles of hydrochloric acid react with 1 mole of lead (ii) nitrate to form 1 mole of lead (ii). To balance a chemical equation, enter an equation of a chemical. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.slideshare.net
Chemistry Lead(Ii) Nitrate Reacts With Hydrochloric Acid In a double replacement reaction, the cations. Fe + hcl = h2 + fecl2 Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. To balance a chemical equation,. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED The unbalanced reaction between lead (II) nitrate and hydrochloric acid is shown below Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. In a double replacement reaction, the cations. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. In our balanced reaction, the. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.
From www.youtube.com
Double displacement HCl + Pb(NO3)2 Hydrochloric acid + Lead(ii) nitrate Precipitation Lead(Ii) Nitrate Reacts With Hydrochloric Acid Fe + hcl = h2 + fecl2 The reaction between lead (ii) nitrate and hydrochloric acid is a double replacement reaction, also called a double displacement reaction. Identify the reactants and products in the given chemical equation, then check if any of the products form a solid precipitate. To balance a chemical equation, enter an equation of a chemical reaction. Lead(Ii) Nitrate Reacts With Hydrochloric Acid.