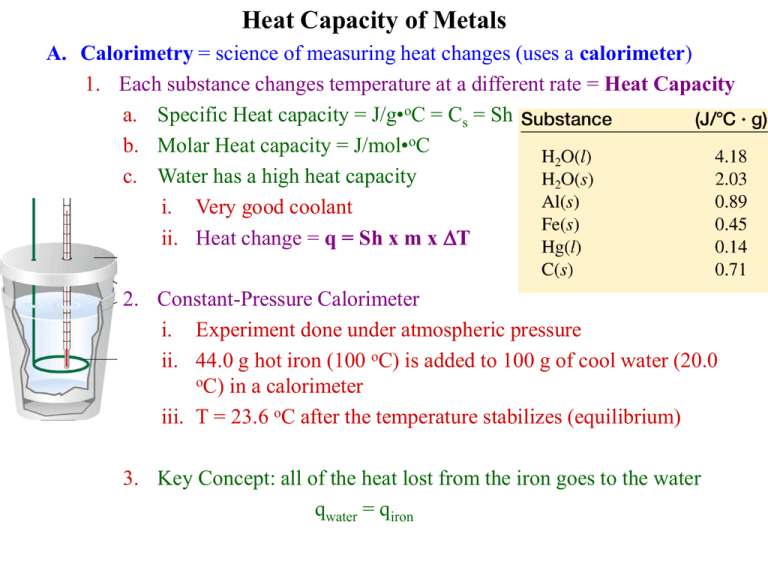
Calorimetry Lab Unknown Metal . When the contents of the calorimeter are. _____ identification of an unknown metal: In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. We will then identify the 5. For example, when a hot piece of metal (the system ) is In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. What is the specific heat of the metal? In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. The device used to measure heat flow is called a calorimeter. Then the metal was transferred to an enclosed styrofoam cup containing room.
from janiyahabbgates.blogspot.com
For example, when a hot piece of metal (the system ) is The device used to measure heat flow is called a calorimeter. We will then identify the 5. What is the specific heat of the metal? Then the metal was transferred to an enclosed styrofoam cup containing room. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. _____ identification of an unknown metal: When the contents of the calorimeter are.
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates
Calorimetry Lab Unknown Metal In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. What is the specific heat of the metal? _____ identification of an unknown metal: We will then identify the 5. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. The device used to measure heat flow is called a calorimeter. In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. When the contents of the calorimeter are. For example, when a hot piece of metal (the system ) is Then the metal was transferred to an enclosed styrofoam cup containing room.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Calorimetry Lab Unknown Metal When the contents of the calorimeter are. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. Then the metal was transferred to an enclosed styrofoam cup containing room. We will then identify the 5. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved Lab 4 Calorimetry An unknown metal can be identified Calorimetry Lab Unknown Metal In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. When the contents of the calorimeter are. We will then identify the 5. In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. Then the metal was transferred. Calorimetry Lab Unknown Metal.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Unknown Metal _____ identification of an unknown metal: In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. The device used to measure heat flow is called a calorimeter. In. Calorimetry Lab Unknown Metal.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Calorimetry Lab Unknown Metal What is the specific heat of the metal? The device used to measure heat flow is called a calorimeter. Then the metal was transferred to an enclosed styrofoam cup containing room. When the contents of the calorimeter are. We will then identify the 5. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved PreLab Experiment 8; Calorimetry 1. An unknown metal Calorimetry Lab Unknown Metal Then the metal was transferred to an enclosed styrofoam cup containing room. We will then identify the 5. The device used to measure heat flow is called a calorimeter. _____ identification of an unknown metal: In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In the calorimetry lab, an unknown. Calorimetry Lab Unknown Metal.
From claire-chapter.blogspot.com
How To Identify An Unknown Metal Using Calorimetry 43+ Pages Solution Calorimetry Lab Unknown Metal In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. For example, when a hot piece of metal (the system ) is What is the specific heat of. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved Calorimetry Dry Lab Data Unknown Metal for Part A Calorimetry Lab Unknown Metal What is the specific heat of the metal? Then the metal was transferred to an enclosed styrofoam cup containing room. _____ identification of an unknown metal: In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. We will then identify the 5. In the calorimetry lab, an. Calorimetry Lab Unknown Metal.
From ar.inspiredpencil.com
Calorimetry Calorimetry Lab Unknown Metal When the contents of the calorimeter are. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. _____ identification of an unknown metal: In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In today's lab, we will use experimental calorimetry to. Calorimetry Lab Unknown Metal.
From studylib.net
Chemistry 1 Unit 2 Calorimetry Lab Calorimetry Lab Unknown Metal In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. For example, when a hot piece of metal (the system ) is The device used to measure heat flow is called a calorimeter. Then the metal was transferred to an enclosed styrofoam cup containing room. What is. Calorimetry Lab Unknown Metal.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Lab Unknown Metal In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. For example, when a hot piece of metal (the system ) is We will then identify the 5.. Calorimetry Lab Unknown Metal.
From users.highland.edu
Calorimetry Calorimetry Lab Unknown Metal In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals.. Calorimetry Lab Unknown Metal.
From www.alamy.com
A beaker of water and a numbered test tube containing an unknown metal Calorimetry Lab Unknown Metal We will then identify the 5. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. Then the metal was transferred to an enclosed styrofoam cup containing room. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. _____ identification of an unknown. Calorimetry Lab Unknown Metal.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Lab Unknown Metal The device used to measure heat flow is called a calorimeter. What is the specific heat of the metal? We will then identify the 5. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. Then the metal was transferred to an enclosed styrofoam cup containing room. _____ identification of an. Calorimetry Lab Unknown Metal.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Lab Unknown Metal The device used to measure heat flow is called a calorimeter. _____ identification of an unknown metal: In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. In your lab investigation you are. Calorimetry Lab Unknown Metal.
From ar.inspiredpencil.com
Specific Heat Of Metals Lab Calorimetry Lab Unknown Metal When the contents of the calorimeter are. _____ identification of an unknown metal: For example, when a hot piece of metal (the system ) is The device used to measure heat flow is called a calorimeter. We will then identify the 5. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals.. Calorimetry Lab Unknown Metal.
From betterlesson.com
Ninth grade Lesson Identifying an Unknown Metal BetterLesson Calorimetry Lab Unknown Metal What is the specific heat of the metal? We will then identify the 5. When the contents of the calorimeter are. In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container. Calorimetry Lab Unknown Metal.
From www.alamy.com
A digital thermometer is used to check the temperature of an unknown Calorimetry Lab Unknown Metal In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. _____ identification of an unknown metal: For example, when a hot piece of metal (the system ) is In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. In the calorimetry lab, an. Calorimetry Lab Unknown Metal.
From claire-chapter.blogspot.com
How To Identify An Unknown Metal Using Calorimetry 43+ Pages Solution Calorimetry Lab Unknown Metal Then the metal was transferred to an enclosed styrofoam cup containing room. The device used to measure heat flow is called a calorimeter. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using. Calorimetry Lab Unknown Metal.
From www.transtutors.com
(Solved) Calorimetry Lab calculate the specific heat capacity of the Calorimetry Lab Unknown Metal In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. What is the specific heat of the metal? For example, when a hot piece of metal (the system ) is We will then identify the 5. The device used to measure heat flow is called a calorimeter.. Calorimetry Lab Unknown Metal.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Calorimetry Lab Unknown Metal In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. For example, when a hot piece of metal (the system ) is In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. What is the specific heat of the metal? In a calorimetry. Calorimetry Lab Unknown Metal.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Lab Unknown Metal For example, when a hot piece of metal (the system ) is We will then identify the 5. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. _____ identification of an unknown metal: In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved Calorimetry Dry Lab Data Unknown Metal for Part A Calorimetry Lab Unknown Metal We will then identify the 5. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. What is the specific heat of the metal? For example, when a hot piece of metal (the system ) is When the contents of the calorimeter are. In your lab investigation you are required to. Calorimetry Lab Unknown Metal.
From studylib.net
LAB FOUR Specific Heat of a Metal Objective Introduction Calorimetry Lab Unknown Metal When the contents of the calorimeter are. Then the metal was transferred to an enclosed styrofoam cup containing room. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. We will then identify the 5. What is the specific heat of the metal? _____ identification of an unknown metal: The device. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved Lab 4 Calorimetry An unknown metal can be identified Calorimetry Lab Unknown Metal What is the specific heat of the metal? The device used to measure heat flow is called a calorimeter. When the contents of the calorimeter are. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. For example, when a hot piece of metal (the system ) is In a calorimetry experiment,. Calorimetry Lab Unknown Metal.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Unknown Metal The device used to measure heat flow is called a calorimeter. What is the specific heat of the metal? In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. For example, when a hot piece of metal (the system ) is Then the metal was transferred to. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved Lab 4 Calorimetry An unknown metal can be identified Calorimetry Lab Unknown Metal We will then identify the 5. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. When the contents of the calorimeter are. What is the specific heat of the metal? In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. _____. Calorimetry Lab Unknown Metal.
From www.chegg.com
Solved IV. Specific Heat Capacity of an Unknown Metal Volume Calorimetry Lab Unknown Metal We will then identify the 5. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. When the contents of the calorimeter are. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In today's lab, we will use experimental calorimetry to. Calorimetry Lab Unknown Metal.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Calorimetry Lab Unknown Metal In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. What is the specific heat of the metal? In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. Then the metal was transferred to an enclosed styrofoam cup. Calorimetry Lab Unknown Metal.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Unknown Metal Then the metal was transferred to an enclosed styrofoam cup containing room. What is the specific heat of the metal? In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. _____ identification of an unknown metal: We will then identify the 5. In a calorimetry experiment, heat is transferred from one. Calorimetry Lab Unknown Metal.
From saylordotorg.github.io
Calorimetry Calorimetry Lab Unknown Metal For example, when a hot piece of metal (the system ) is The device used to measure heat flow is called a calorimeter. In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c.. Calorimetry Lab Unknown Metal.
From www.youtube.com
Calorimetry Lab Unknown Metal Heat Capcity YouTube Calorimetry Lab Unknown Metal What is the specific heat of the metal? The device used to measure heat flow is called a calorimeter. We will then identify the 5. _____ identification of an unknown metal: In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. Then the metal was transferred to. Calorimetry Lab Unknown Metal.
From claire-chapter.blogspot.com
How To Identify An Unknown Metal Using Calorimetry 43+ Pages Solution Calorimetry Lab Unknown Metal In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. For example, when a hot piece of metal (the system ) is In today's lab, we will use experimental calorimetry to calculate the unique specific heat values of 5 metals. In a calorimetry experiment, heat is transferred from one object to. Calorimetry Lab Unknown Metal.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Calorimetry Lab Unknown Metal In your lab investigation you are required to find the specific heat capacity of four unknown metals and, using the table of specific. What is the specific heat of the metal? For example, when a hot piece of metal (the system ) is The device used to measure heat flow is called a calorimeter. In the calorimetry lab, an unknown. Calorimetry Lab Unknown Metal.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Unknown Metal _____ identification of an unknown metal: We will then identify the 5. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. In the calorimetry lab, an unknown metal was placed in a test tube surrounded by boiling water at 100.00∘c. When the contents of the calorimeter are. For example, when. Calorimetry Lab Unknown Metal.
From www.numerade.com
SOLVED Experiment 25 Report Sheet Calorimetry Dale Lab Sec Name Desk Calorimetry Lab Unknown Metal _____ identification of an unknown metal: What is the specific heat of the metal? We will then identify the 5. In a calorimetry experiment, heat is transferred from one object to another inside an insulated container called a calorimeter. The device used to measure heat flow is called a calorimeter. For example, when a hot piece of metal (the system. Calorimetry Lab Unknown Metal.