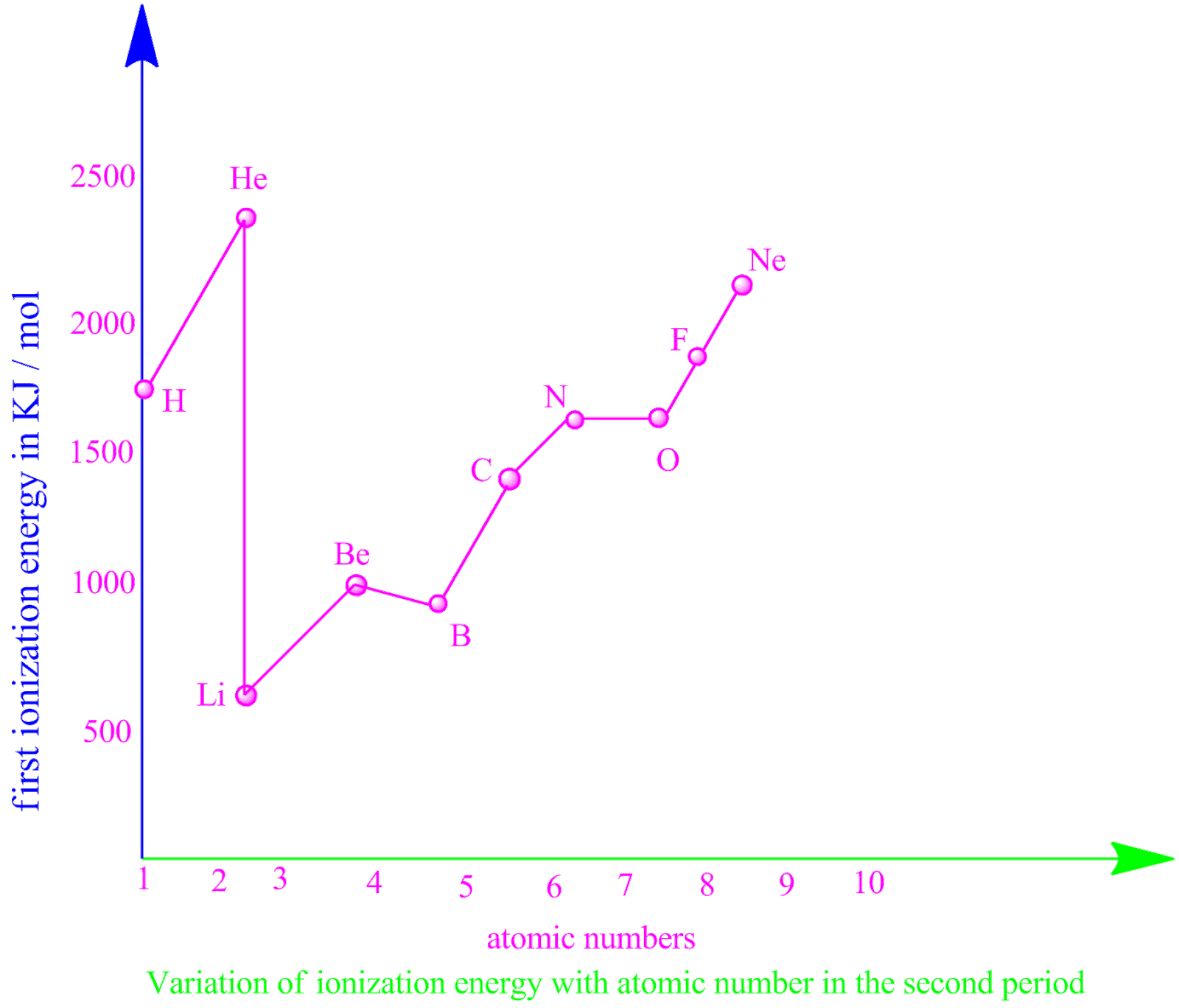
Second Ionization Energy Of Chlorine Equation . Third ionization energy, i3 (general element, a):. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. the general equation for the ionization energy is as follows. second ionization energy, i2 (general element, a): Cl + ie → cl+ + e− ie = 12.9676. second ionisation energy is defined by the equation: The higher the value of the ionization energy, the. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. A1 + (g) → a2 + (g) + e −.
from chemisfast.blogspot.com
remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. Cl + ie → cl+ + e− ie = 12.9676. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. Third ionization energy, i3 (general element, a):. The higher the value of the ionization energy, the. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. second ionization energy, i2 (general element, a): the general equation for the ionization energy is as follows.
What is ionization energy and why second ionization energy is greater
Second Ionization Energy Of Chlorine Equation second ionisation energy is defined by the equation: The higher the value of the ionization energy, the. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. the general equation for the ionization energy is as follows. second ionization energy, i2 (general element, a): a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. Cl + ie → cl+ + e− ie = 12.9676. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. A1 + (g) → a2 + (g) + e −. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. Third ionization energy, i3 (general element, a):. second ionisation energy is defined by the equation:
From www.tes.com
Ionisation Energy AS Level Teaching Resources Second Ionization Energy Of Chlorine Equation we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. second ionization energy, i2 (general element, a): The higher the value of the ionization energy, the. remember that the first ionization energy (ie 1) is the energy required to remove the most. Second Ionization Energy Of Chlorine Equation.
From www.doubtnut.com
The first ΔiH1 and the second ΔiH ionization enthalpies in kJ mol Second Ionization Energy Of Chlorine Equation Cl + ie → cl+ + e− ie = 12.9676. the general equation for the ionization energy is as follows. The higher the value of the ionization energy, the. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. a chlorine atom,. Second Ionization Energy Of Chlorine Equation.
From ecurrencythailand.com
Which Equation Corresponds To The Electron Affinity Of Chlorine? Top Second Ionization Energy Of Chlorine Equation the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. the general equation for the ionization energy is as follows. A1 + (g) → a2 + (g) + e −. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. The higher the. Second Ionization Energy Of Chlorine Equation.
From lessonmagicthirties.z14.web.core.windows.net
How To Write An Ionization Energy Equation Second Ionization Energy Of Chlorine Equation Cl + ie → cl+ + e− ie = 12.9676. second ionization energy, i2 (general element, a): Third ionization energy, i3 (general element, a):. the general equation for the ionization energy is as follows. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. A1 + (g) → a2 + (g) +. Second Ionization Energy Of Chlorine Equation.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Second Ionization Energy Of Chlorine Equation we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. Cl + ie → cl+ + e− ie = 12.9676. second ionisation energy is defined by the equation: the second ionisation energy is the energy required when one mole of gaseous ions. Second Ionization Energy Of Chlorine Equation.
From www.tessshebaylo.com
Equation For Second Ionisation Energy Of Calcium Tessshebaylo Second Ionization Energy Of Chlorine Equation the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. Third ionization energy, i3 (general element, a):. Cl + ie → cl+ + e− ie = 12.9676. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral.. Second Ionization Energy Of Chlorine Equation.
From www.youtube.com
Example Trend in Second Ionization Energy YouTube Second Ionization Energy Of Chlorine Equation a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. second ionization energy, i2 (general element, a): A1 + (g) → a2 + (g) + e −. remember that the first ionization. Second Ionization Energy Of Chlorine Equation.
From www.slideserve.com
PPT IONISATION ENERGY PowerPoint Presentation, free download ID3500637 Second Ionization Energy Of Chlorine Equation remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. A1 + (g) → a2 + (g) + e −. Third ionization energy, i3 (general element, a):. second ionisation energy is defined by the equation: The higher the value of the ionization energy, the. the. Second Ionization Energy Of Chlorine Equation.
From ck12.org
When we look again at the table, we can see that the ionization energy Second Ionization Energy Of Chlorine Equation second ionization energy, i2 (general element, a): remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. Cl + ie → cl+ + e− ie = 12.9676. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. Third ionization energy,. Second Ionization Energy Of Chlorine Equation.
From www.youtube.com
First and second ionization energy Atomic structure and properties Second Ionization Energy Of Chlorine Equation A1 + (g) → a2 + (g) + e −. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. second ionisation energy is defined by the equation: the general equation for the ionization energy is as follows. The higher the value of the ionization energy, the. Cl + ie → cl+. Second Ionization Energy Of Chlorine Equation.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Second Ionization Energy Of Chlorine Equation the general equation for the ionization energy is as follows. Third ionization energy, i3 (general element, a):. A1 + (g) → a2 + (g) + e −. The higher the value of the ionization energy, the. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. we can. Second Ionization Energy Of Chlorine Equation.
From www.numerade.com
SOLVEDWrite equations that show the processes that describe the first Second Ionization Energy Of Chlorine Equation A1 + (g) → a2 + (g) + e −. the general equation for the ionization energy is as follows. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. the second ionisation energy is the energy required when one mole of gaseous ions with. Second Ionization Energy Of Chlorine Equation.
From byjus.com
29. The first,second and third ionization energies are 578,1187and 2745 Second Ionization Energy Of Chlorine Equation the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. Cl + ie → cl+ + e− ie = 12.9676. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. Third ionization energy, i3. Second Ionization Energy Of Chlorine Equation.
From chemisfast.blogspot.com
What is ionization energy and why second ionization energy is greater Second Ionization Energy Of Chlorine Equation The higher the value of the ionization energy, the. Third ionization energy, i3 (general element, a):. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. second ionization energy, i2 (general element, a): a chlorine atom, for example, requires the following ionization energy to remove the outermost electron.. Second Ionization Energy Of Chlorine Equation.
From www.chemistrylearner.com
Ionization Energy Definition, Chart & Periodic Table Trend Second Ionization Energy Of Chlorine Equation second ionization energy, i2 (general element, a): the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. The higher the value of the ionization energy, the. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according. Second Ionization Energy Of Chlorine Equation.
From fyofgpkgr.blob.core.windows.net
Electron Affinity Of Chlorine Equation at Marilyn Burton blog Second Ionization Energy Of Chlorine Equation second ionisation energy is defined by the equation: Third ionization energy, i3 (general element, a):. Cl + ie → cl+ + e− ie = 12.9676. the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. we can define a first ionization energy (i1), a second ionization energy (i2),. Second Ionization Energy Of Chlorine Equation.
From www.tessshebaylo.com
What Is The Equation For Second Ionisation Energy Of Oxygen Tessshebaylo Second Ionization Energy Of Chlorine Equation a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. Cl + ie → cl+ + e− ie = 12.9676. the general equation for the ionization energy is as follows. Third ionization energy, i3 (general element, a):. remember that the first ionization energy (ie 1) is the energy required to remove the. Second Ionization Energy Of Chlorine Equation.
From www.animalia-life.club
Second Ionization Energy Periodic Table Second Ionization Energy Of Chlorine Equation The higher the value of the ionization energy, the. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. Third ionization. Second Ionization Energy Of Chlorine Equation.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Second Ionization Energy Of Chlorine Equation a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. second ionization energy, i2 (general element, a): the general equation for the ionization energy is as follows. Third ionization energy, i3 (general element, a):. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an. Second Ionization Energy Of Chlorine Equation.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Second Ionization Energy Of Chlorine Equation we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. The higher the value of the ionization energy, the. Cl + ie → cl+ + e− ie = 12.9676. the general equation for the ionization energy is as follows. second ionisation energy. Second Ionization Energy Of Chlorine Equation.
From hxewfgvpo.blob.core.windows.net
Chlorine Ionic Reaction at Howard Saad blog Second Ionization Energy Of Chlorine Equation a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. the general equation for the ionization energy is as follows. A1 + (g) → a2 + (g) + e −. second ionisation. Second Ionization Energy Of Chlorine Equation.
From www.numerade.com
When chlorine gains an electron to a chloride ion with a 21 Second Ionization Energy Of Chlorine Equation second ionization energy, i2 (general element, a): The higher the value of the ionization energy, the. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. the general equation for the ionization energy is as follows. A1 + (g) → a2 + (g) + e. Second Ionization Energy Of Chlorine Equation.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Second Ionization Energy Of Chlorine Equation A1 + (g) → a2 + (g) + e −. Third ionization energy, i3 (general element, a):. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. remember that the first ionization energy (ie 1) is the energy required to remove the most. Second Ionization Energy Of Chlorine Equation.
From www.animalia-life.club
Second Ionization Energy Periodic Table Second Ionization Energy Of Chlorine Equation Cl + ie → cl+ + e− ie = 12.9676. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. second ionisation energy is defined by the equation: The higher the value of the ionization energy, the. a chlorine atom, for example,. Second Ionization Energy Of Chlorine Equation.
From www.numerade.com
SOLVED Write the chemical formula for aluminum chloride Answer Use Second Ionization Energy Of Chlorine Equation the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. Cl + ie → cl+ + e− ie = 12.9676. The higher the value of the ionization energy, the. second ionization energy, i2 (general element, a): the second ionisation energy is the energy required when one mole of. Second Ionization Energy Of Chlorine Equation.
From www.animalia-life.club
Second Ionization Energy Periodic Table Second Ionization Energy Of Chlorine Equation we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. remember that the first ionization energy (ie 1) is the energy required to remove the most loosely bound electron from a neutral. the second ionisation energy is the energy required when one. Second Ionization Energy Of Chlorine Equation.
From fyofgpkgr.blob.core.windows.net
Electron Affinity Of Chlorine Equation at Marilyn Burton blog Second Ionization Energy Of Chlorine Equation second ionisation energy is defined by the equation: Third ionization energy, i3 (general element, a):. Cl + ie → cl+ + e− ie = 12.9676. second ionization energy, i2 (general element, a): the general equation for the ionization energy is as follows. the symbol \(i_1\) stands for the first ionization energy (energy required to take away. Second Ionization Energy Of Chlorine Equation.
From learnah.org
5 Ionisation Energies Second Ionization Energy Of Chlorine Equation Third ionization energy, i3 (general element, a):. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. second ionisation energy is defined by the equation: The higher the value of the ionization energy, the. Cl + ie → cl+ + e− ie =. Second Ionization Energy Of Chlorine Equation.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Second Ionization Energy Of Chlorine Equation we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. the general equation for the ionization energy is as follows. the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. The higher the. Second Ionization Energy Of Chlorine Equation.
From www.sliderbase.com
The Ionization Energy Second Ionization Energy Of Chlorine Equation Cl + ie → cl+ + e− ie = 12.9676. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. second ionisation energy is defined by the equation: second ionization energy, i2 (general element, a): The higher the value of the ionization. Second Ionization Energy Of Chlorine Equation.
From www.slideserve.com
PPT Electronic Structure and the Periodic Table PowerPoint Second Ionization Energy Of Chlorine Equation we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. Third ionization energy, i3 (general element, a):. the general equation for the ionization energy is as follows. The higher the value of the ionization energy, the. the second ionisation energy is the. Second Ionization Energy Of Chlorine Equation.
From www.numerade.com
The value of the second ionization energy of calcium is 1150 KJ/mol Second Ionization Energy Of Chlorine Equation a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. Third ionization energy, i3 (general element, a):. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. Cl + ie → cl+ + e− ie = 12.9676. remember that the first ionization energy. Second Ionization Energy Of Chlorine Equation.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Second Ionization Energy Of Chlorine Equation Cl + ie → cl+ + e− ie = 12.9676. the symbol \(i_1\) stands for the first ionization energy (energy required to take away an electron from a neutral. we can define a first ionization energy (i1), a second ionization energy (i2), and in general an nth ionization energy (in) according to the following. A1 + (g) →. Second Ionization Energy Of Chlorine Equation.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Second Ionization Energy Of Chlorine Equation Cl + ie → cl+ + e− ie = 12.9676. Third ionization energy, i3 (general element, a):. the second ionisation energy is the energy required when one mole of gaseous ions with a single positive charge. second ionisation energy is defined by the equation: The higher the value of the ionization energy, the. remember that the first. Second Ionization Energy Of Chlorine Equation.
From www.slideserve.com
PPT History of the Periodic Table PowerPoint Presentation ID1210134 Second Ionization Energy Of Chlorine Equation The higher the value of the ionization energy, the. second ionisation energy is defined by the equation: the general equation for the ionization energy is as follows. a chlorine atom, for example, requires the following ionization energy to remove the outermost electron. remember that the first ionization energy (ie 1) is the energy required to remove. Second Ionization Energy Of Chlorine Equation.