Titration Of Khp With Naoh . Calculate the percentage of khp in the. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. If the concentration of the khp solution is known accurately and the. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. You will prepare ~0.1 m naoh solution and standardize this solution. The data from the titration is then used to calculate the molarity of the naoh.
from www.numerade.com
If the concentration of the khp solution is known accurately and the. The data from the titration is then used to calculate the molarity of the naoh. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. You will prepare ~0.1 m naoh solution and standardize this solution. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Calculate the percentage of khp in the.
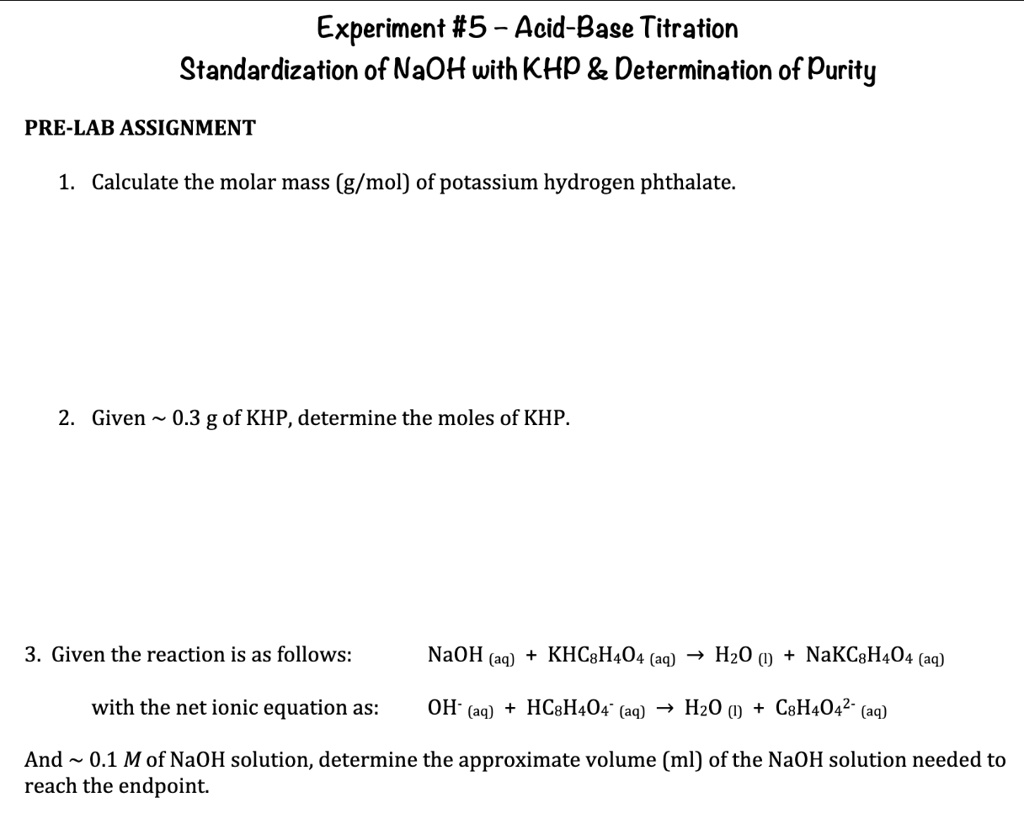
SOLVED Question 3 Please Experiment 5 AcidBase Titration
Titration Of Khp With Naoh add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. If the concentration of the khp solution is known accurately and the. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. Calculate the percentage of khp in the. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. You will prepare ~0.1 m naoh solution and standardize this solution. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. The data from the titration is then used to calculate the molarity of the naoh. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via.
From www.chegg.com
draw a titration curve of NaOH and KHP draw a Titration Of Khp With Naoh to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. You will prepare ~0.1 m naoh solution and standardize this solution. The data from the titration is then used to calculate the molarity of the. Titration Of Khp With Naoh.
From www.chegg.com
Solved Titration of KHP to Determine Concentration of NaOH Titration Of Khp With Naoh to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. If the concentration of the khp solution is known accurately and the. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. Calculate the percentage of khp in the. You will prepare ~0.1 m naoh. Titration Of Khp With Naoh.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Khp With Naoh add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Calculate the percentage of khp in the. the titration of naoh with khp involves adding naoh from the burette to a known. Titration Of Khp With Naoh.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Titration Of Khp With Naoh to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. You will prepare ~0.1 m naoh solution and standardize this solution. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Calculate the percentage of khp in the. the titration of naoh with khp. Titration Of Khp With Naoh.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Khp With Naoh The data from the titration is then used to calculate the molarity of the naoh. If the concentration of the khp solution is known accurately and the. Calculate the percentage of khp in the. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. the titration of naoh with khp involves. Titration Of Khp With Naoh.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Khp With Naoh the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. You will prepare ~0.1 m naoh solution and standardize this solution. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. use the virtual laboratory to standardize an unknown naoh. Titration Of Khp With Naoh.
From www.youtube.com
Mass of KHP to Standardize a NaOH Solution YouTube Titration Of Khp With Naoh You will prepare ~0.1 m naoh solution and standardize this solution. If the concentration of the khp solution is known accurately and the. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate. Titration Of Khp With Naoh.
From www.numerade.com
SOLVEDFor titration of a NaOH solution, HKC8H4O4 (KHP) is used as the Titration Of Khp With Naoh add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. Calculate the percentage of khp in the. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures. Titration Of Khp With Naoh.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Khp With Naoh You will prepare ~0.1 m naoh solution and standardize this solution. If the concentration of the khp solution is known accurately and the. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via.. Titration Of Khp With Naoh.
From www.numerade.com
SOLVEDBonus Question NaOH was standardized by titration against a Titration Of Khp With Naoh use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. add a few drops of phenolphthalein. Titration Of Khp With Naoh.
From www.scribd.com
Laboratory Plan 1 Standardization of Sodium Hydroxide (Naoh) With Titration Of Khp With Naoh use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The data from the titration is then used to calculate the molarity of the naoh. standardization of a naoh solution with potassium hydrogen. Titration Of Khp With Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Of Khp With Naoh the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from the titration is then used to calculate the molarity of the naoh. Calculate the percentage of khp in. Titration Of Khp With Naoh.
From www.youtube.com
Processing Data from Titration of NaOH with KHP YouTube Titration Of Khp With Naoh standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Calculate the percentage of khp in the. You will prepare ~0.1 m naoh solution and standardize this solution. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. The data from the titration. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED 'KHPNaOH titration results phthalic acid from the Calculate Titration Of Khp With Naoh The data from the titration is then used to calculate the molarity of the naoh. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. If the concentration of the khp solution is known accurately and the. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four. Titration Of Khp With Naoh.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Khp With Naoh standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. If the concentration of the khp solution is known accurately and the. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m). Titration Of Khp With Naoh.
From www.chegg.com
Solved calculating the moles of NaOH from titration of KHP Titration Of Khp With Naoh standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. You will prepare ~0.1 m naoh solution. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED Figure 1. KHP and NaOH Titration Curve of pH vs Volume of NaOH Titration Of Khp With Naoh The data from the titration is then used to calculate the molarity of the naoh. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. If the concentration of the khp solution is known accurately. Titration Of Khp With Naoh.
From plot.ly
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Of Khp With Naoh standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. You will prepare ~0.1 m naoh solution and standardize this solution. Calculate the percentage of khp in the. The data from the titration is then used to calculate the molarity of the naoh. to standardize a sodium hydroxide (naoh) solution against. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED In your acidbase titration lab, you performed the titration of Titration Of Khp With Naoh You will prepare ~0.1 m naoh solution and standardize this solution. If the concentration of the khp solution is known accurately and the. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint.. Titration Of Khp With Naoh.
From plotly.com
First Derivative of KHP and NaOH Titration Curve line chart made by Titration Of Khp With Naoh use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. The data from the titration is then used to calculate the molarity of the naoh. standardization of a naoh solution with potassium hydrogen phthalate. Titration Of Khp With Naoh.
From www.scribd.com
Calculations. Titration Lab NaOH With Standardized Solution of KHP Titration Of Khp With Naoh standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. standardization of a naoh solution. Titration Of Khp With Naoh.
From www.youtube.com
Standardization of NaOH by titration using KHP YouTube Titration Of Khp With Naoh to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. You will prepare ~0.1 m naoh solution and standardize this solution. Calculate the percentage of khp in the. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. add a few drops of phenolphthalein. Titration Of Khp With Naoh.
From www.vrogue.co
Standardization Of Naoh With A Khp Solution Acid Base vrogue.co Titration Of Khp With Naoh Calculate the percentage of khp in the. You will prepare ~0.1 m naoh solution and standardize this solution. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. If the concentration of the khp solution. Titration Of Khp With Naoh.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Khp With Naoh add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The data from the titration is then used to calculate the molarity of the naoh. the titration of naoh with khp involves. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED Calculating the Concentration of a Sodium Hydroxide Solution Titration Of Khp With Naoh use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. Calculate the percentage. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED Preparing standard KHP and titration with NaOH Molar mass of Titration Of Khp With Naoh standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. If the concentration of the khp solution is known accurately and the. The data from the titration is then used to calculate the molarity of the naoh. add a few drops of phenolphthalein and titrate each sample with standardized naoh to. Titration Of Khp With Naoh.
From www.chegg.com
Solved Titration of KHP to Determine Concentration of NaOH Titration Of Khp With Naoh to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The data from the titration is then. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED TITRATION 1 Mass KHP 2.073 g Volume of KHP Solution 1O0.0 mL Titration Of Khp With Naoh The data from the titration is then used to calculate the molarity of the naoh. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. add a few drops of phenolphthalein and titrate each sample with standardized naoh to a faint pink endpoint. Calculate the percentage of khp in the. You will. Titration Of Khp With Naoh.
From www.chegg.com
Solved Titration of KHP to Determine Concentration of NaOH Titration Of Khp With Naoh standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Calculate the percentage of khp in the. the titration of naoh with khp involves adding naoh from the burette to a known mass. Titration Of Khp With Naoh.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Khp With Naoh You will prepare ~0.1 m naoh solution and standardize this solution. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from the titration is then used to calculate the molarity of the. Titration Of Khp With Naoh.
From studylib.net
CHM 115 Lab 3 Titration Standardize NaOH/Determine impure KHP Titration Of Khp With Naoh If the concentration of the khp solution is known accurately and the. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. The data from the titration is then used to calculate the molarity of the naoh. to standardize a sodium hydroxide (naoh) solution against a primary standard. Titration Of Khp With Naoh.
From www.chegg.com
Solved Titration of NaOH with KHP primary standard Molar Titration Of Khp With Naoh The data from the titration is then used to calculate the molarity of the naoh. to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. Calculate the percentage of khp in the. You will prepare ~0.1 m naoh solution and standardize this solution. standardize a sodium hydroxide (naoh) solution using titration of. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED Question 3 Please Experiment 5 AcidBase Titration Titration Of Khp With Naoh use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. You will prepare ~0.1 m naoh solution and standardize this solution. the titration of naoh with khp involves adding naoh from the burette. Titration Of Khp With Naoh.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Titration Of Khp With Naoh the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. add a. Titration Of Khp With Naoh.
From www.chegg.com
Solved For each titration of KHP with NaOH solution, Titration Of Khp With Naoh to standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. The data from the titration is then used to calculate the molarity of the naoh. Calculate the percentage of khp in the.. Titration Of Khp With Naoh.