Evaporation Exothermic . Evaporation changes liquid water to water vapor. All phase changes are accompanied by changes in the energy of a system. Exothermic vs endothermic chemical reactions. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. The sign of q q for an endothermic process is positive because the system is gaining heat. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. Since the liquid water molecules must absorb. Is evaporation endothermic or exothermic? The quantity of heat for a process is represented by the letter q q. Changes of state are examples of phase changes, or phase transitions. Energy is absorbed from the pan to. This energy comes from the surrounding air, which cools as the water evaporates. Define endothermic and exothermic reactions.
from slideplayer.com
Is evaporation endothermic or exothermic? The sign of q q for an endothermic process is positive because the system is gaining heat. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Describe how heat is transferred in endothermic and exothermic reactions. Sweating cools a person down as water draws heat to change into gas form. Since the liquid water molecules must absorb. All phase changes are accompanied by changes in the energy of a system. Energy from the sun causes water to evaporate. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q.
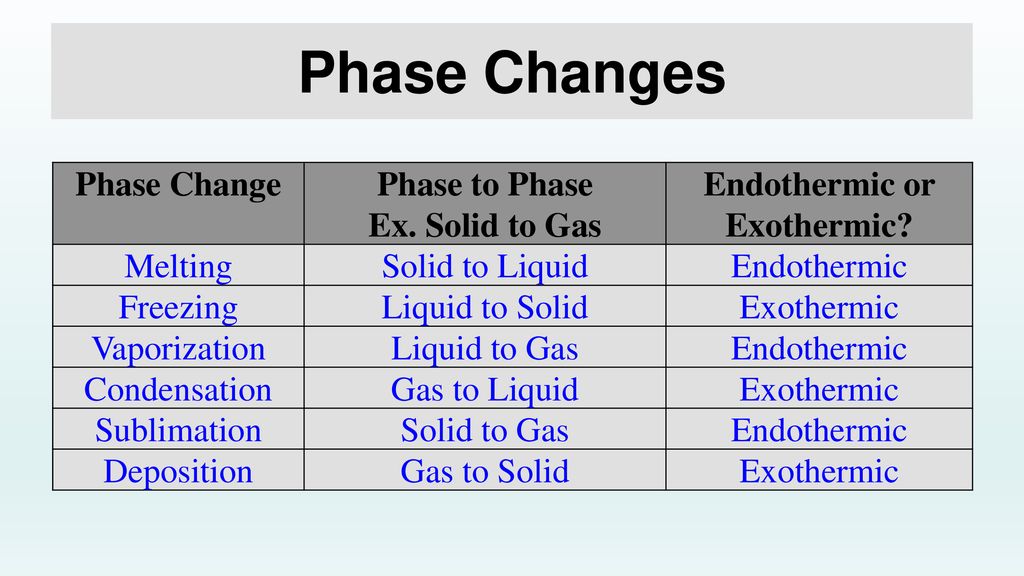
Changes of State Melting, Freezing, Vaporization, Evaporation, Condensation, Sublimation
Evaporation Exothermic The sign of q q for an endothermic process is positive because the system is gaining heat. Evaporation changes liquid water to water vapor. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. The sign of q q for an endothermic process is positive because the system is gaining heat. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Since the liquid water molecules must absorb. This energy comes from the surrounding air, which cools as the water evaporates. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. Energy is absorbed from the pan to. Define endothermic and exothermic reactions. Sweating cools a person down as water draws heat to change into gas form. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. All phase changes are accompanied by changes in the energy of a system. The quantity of heat for a process is represented by the letter q q.
From jacksofscience.com
Is Evaporation Endothermic or Exothermic? Jacks Of Science Evaporation Exothermic Changes of state are examples of phase changes, or phase transitions. The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. All phase changes are accompanied by changes in the energy of a system. Energy is absorbed from the pan to. Is evaporation endothermic or exothermic? The quantity of heat for a. Evaporation Exothermic.
From slideplayer.com
LiquidVapor Equilibrium ppt download Evaporation Exothermic Evaporation changes liquid water to water vapor. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Sweating cools a person down as water draws heat to change into gas form. Exothermic vs endothermic chemical reactions. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous. Evaporation Exothermic.
From www.pearson.com
Endothermic & Exothermic Reactions Channels for Pearson+ Evaporation Exothermic Energy is absorbed from the pan to. Define endothermic and exothermic reactions. Sweating cools a person down as water draws heat to change into gas form. Energy from the sun causes water to evaporate. Changes of state are examples of phase changes, or phase transitions. Describe how heat is transferred in endothermic and exothermic reactions. Evaporation, process by which an. Evaporation Exothermic.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Evaporation Exothermic A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Energy from the sun causes water to evaporate. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. The process of evaporation is endothermic, meaning that it. Evaporation Exothermic.
From www.numerade.com
SOLVED Explain changes in states of matter in terms of exothermic and endothermic activity 5 Evaporation Exothermic Exothermic vs endothermic chemical reactions. The sign of q q for an endothermic process is positive because the system is gaining heat. All phase changes are accompanied by changes in the energy of a system. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate.. Evaporation Exothermic.
From www.animalia-life.club
Evaporation Examples For Kids Evaporation Exothermic Energy from the sun causes water to evaporate. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Since the liquid water molecules must absorb. The sign of q q for an endothermic process is positive because the system is gaining heat. Changes of state. Evaporation Exothermic.
From www.diffzy.com
Exothermic vs. Endothermic Reactions What's the Difference (With Table) Evaporation Exothermic All phase changes are accompanied by changes in the energy of a system. The quantity of heat for a process is represented by the letter q q. Since the liquid water molecules must absorb. Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. A chemical reaction or physical change is exothermic if heat is. Evaporation Exothermic.
From socratic.org
Why is vaporization endothermic? Why is condensation exothermic? Socratic Evaporation Exothermic Changes of state are examples of phase changes, or phase transitions. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Describe how heat is transferred in endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. Energy from the sun. Evaporation Exothermic.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Evaporation Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Define endothermic and exothermic reactions. Energy is absorbed from the pan to. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. All phase. Evaporation Exothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Evaporation Exothermic The sign of q q for an endothermic process is positive because the system is gaining heat. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Is evaporation endothermic or exothermic? A chemical reaction or physical change is exothermic if heat is released by. Evaporation Exothermic.
From lavelle.chem.ucla.edu
melting CHEMISTRY COMMUNITY Evaporation Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Define endothermic and exothermic reactions. Evaporation changes liquid water to water vapor. Since the liquid water molecules must absorb. It is also how liquid. Evaporation Exothermic.
From mappingmemories.ca
La risa Limitado bendición is sublimation endothermic Abundantemente trompeta virtual Evaporation Exothermic Evaporation changes liquid water to water vapor. Is evaporation endothermic or exothermic? Exothermic vs endothermic chemical reactions. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. This energy comes from the surrounding air, which cools as the water evaporates. Evaporation, process by which an element or compound transitions from its liquid. Evaporation Exothermic.
From www.slideserve.com
PPT Activity 8 Chemical Energy PowerPoint Presentation, free download ID6850804 Evaporation Exothermic Exothermic vs endothermic chemical reactions. The sign of q q for an endothermic process is positive because the system is gaining heat. This energy comes from the surrounding air, which cools as the water evaporates. Describe how heat is transferred in endothermic and exothermic reactions. It is also how liquid water enters the atmosphere as water vapor, which is an. Evaporation Exothermic.
From courses.lumenlearning.com
Exothermic and Endothermic Processes Introduction to Chemistry Evaporation Exothermic Energy is absorbed from the pan to. Describe how heat is transferred in endothermic and exothermic reactions. This energy comes from the surrounding air, which cools as the water evaporates. Is evaporation endothermic or exothermic? The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. Evaporation changes liquid water to water vapor.. Evaporation Exothermic.
From slideplayer.com
Intermolecular forces ppt download Evaporation Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Describe how heat is transferred in endothermic and exothermic reactions. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Changes of state are examples of phase changes, or phase transitions. Energy is absorbed from the pan. Evaporation Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Evaporation Exothermic This energy comes from the surrounding air, which cools as the water evaporates. Describe how heat is transferred in endothermic and exothermic reactions. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Since the liquid water molecules must absorb. Exothermic vs endothermic chemical reactions. A chemical reaction or. Evaporation Exothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Evaporation Exothermic The sign of q q for an endothermic process is positive because the system is gaining heat. Changes of state are examples of phase changes, or phase transitions. Exothermic vs endothermic chemical reactions. Define endothermic and exothermic reactions. Since the liquid water molecules must absorb. It is also how liquid water enters the atmosphere as water vapor, which is an. Evaporation Exothermic.
From in.pinterest.com
Evaporation Definition, Meaning, Water Cycle, Endo or Exothermic? Physical or Chemical Change Evaporation Exothermic All phase changes are accompanied by changes in the energy of a system. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Sweating cools a person down as water draws heat to change into gas form. Energy from the sun causes water to evaporate. Describe how heat is. Evaporation Exothermic.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID4497767 Evaporation Exothermic Energy from the sun causes water to evaporate. The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Define endothermic and exothermic reactions. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and. Evaporation Exothermic.
From www.slideserve.com
PPT Activity 8 Chemical Energy PowerPoint Presentation, free download ID6850804 Evaporation Exothermic Since the liquid water molecules must absorb. Define endothermic and exothermic reactions. Is evaporation endothermic or exothermic? Changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. Energy is absorbed from the pan to. This energy comes from the surrounding air, which cools as the water. Evaporation Exothermic.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Board, Class 8. Evaporation Exothermic Sweating cools a person down as water draws heat to change into gas form. Exothermic vs endothermic chemical reactions. Evaporation changes liquid water to water vapor. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Describe how heat is transferred in endothermic and exothermic reactions. It is also. Evaporation Exothermic.
From www.numerade.com
SOLVED Question 35 (2 points) Which pair of processes are both Exothermic. Sublimation and Evaporation Exothermic The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. All phase changes are accompanied by changes in the energy of a system. This energy comes from the surrounding air, which cools as the water evaporates. The sign of q q for an endothermic process is positive because the system is gaining. Evaporation Exothermic.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Evaporation Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Energy from the sun causes water to evaporate. Changes of state are examples of phase changes, or phase transitions. Describe how heat is transferred in endothermic and exothermic reactions. Evaporation changes liquid water to water vapor. The process of evaporation is endothermic, meaning that it. Evaporation Exothermic.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing Evaporation Exothermic A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Energy from the sun causes water to evaporate. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Describe how heat is transferred in endothermic and exothermic. Evaporation Exothermic.
From www.chemistrylearner.com
Exothermic Reaction Definition, Equation, and Examples Evaporation Exothermic The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. Since the liquid water molecules must absorb. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Define endothermic and exothermic reactions. Evaporation, process by which an element or compound transitions from its liquid state to its. Evaporation Exothermic.
From www.chegg.com
Solved 9. Which process is exothermic? a) Evaporation of Evaporation Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Exothermic vs endothermic chemical reactions. Since the liquid water molecules must absorb. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Define endothermic and exothermic reactions. Evaporation, process by which an element or compound transitions from its liquid state to its. Evaporation Exothermic.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Evaporation Exothermic Evaporation changes liquid water to water vapor. The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Energy is absorbed from the pan to. Since the liquid. Evaporation Exothermic.
From vectormine.com
Evaporation vector illustration VectorMine Evaporation Exothermic The process of evaporation is endothermic, meaning that it requires energy to convert liquid water into water vapor. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. The sign of q q for an endothermic process is positive because the system is gaining heat. Evaporation changes liquid water. Evaporation Exothermic.
From stock.adobe.com
Physical states of matter.Solid, liquid and gas.Melting, freezing evaporation, condensation Evaporation Exothermic All phase changes are accompanied by changes in the energy of a system. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Exothermic vs endothermic chemical reactions. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and. Evaporation Exothermic.
From higheducationlearning.com
Is Sublimation Endothermic Or Exothermic Process? » Education Tips Evaporation Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. Sweating cools a person down as water draws heat to change into gas form. Define endothermic and exothermic reactions. Since the liquid water molecules must absorb. Evaporation, process by which an element or compound. Evaporation Exothermic.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Evaporation Exothermic Since the liquid water molecules must absorb. Is evaporation endothermic or exothermic? The sign of q q for an endothermic process is positive because the system is gaining heat. Sweating cools a person down as water draws heat to change into gas form. Describe how heat is transferred in endothermic and exothermic reactions. Exothermic vs endothermic chemical reactions. Evaporation changes. Evaporation Exothermic.
From jacksofscience.com
Is Evaporation Endothermic or Exothermic? Jacks Of Science Evaporation Exothermic The quantity of heat for a process is represented by the letter q q. Energy from the sun causes water to evaporate. This energy comes from the surrounding air, which cools as the water evaporates. Evaporation changes liquid water to water vapor. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below. Evaporation Exothermic.
From chem.libretexts.org
11.E Liquids and Intermolecular Forces (Exercises) Chemistry LibreTexts Evaporation Exothermic The sign of q q for an endothermic process is positive because the system is gaining heat. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Energy from the sun causes water to evaporate. Changes of state are examples of phase changes, or phase. Evaporation Exothermic.
From slideplayer.com
Changes of State Melting, Freezing, Vaporization, Evaporation, Condensation, Sublimation Evaporation Exothermic Sweating cools a person down as water draws heat to change into gas form. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Define endothermic and exothermic reactions. Energy is absorbed from the pan to. Energy from the sun causes water to evaporate. Changes. Evaporation Exothermic.
From www.numerade.com
SOLVEDEvaporation of water is (1) An exothermic change (2) An endothermic change (3) A process Evaporation Exothermic Since the liquid water molecules must absorb. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Is evaporation endothermic or exothermic? This energy comes from the surrounding air, which cools as the water evaporates. The sign of q q for an endothermic process is positive because the system is gaining heat. The quantity of. Evaporation Exothermic.