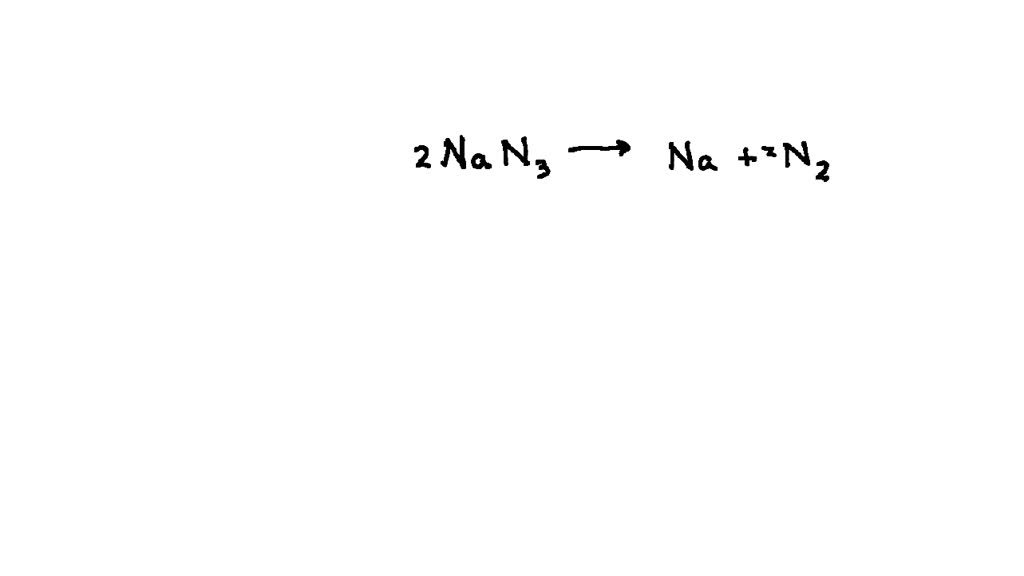Chemical Reaction Used In Airbags . This week on reactions, we’re. The chemical at the heart of. The answer would be found in a fascinating chemical called sodium azide, nan3. Most cars have airbags built into the dashboard and steering. Did you know that a really fast chemical reaction makes riding in a car safer? Manufacturers use different chemical stews to fill their airbags. Guanidinium nitrate, plus a copper nitrate oxidizer. When this substance is ignited by a spark it releases nitrogen gas which can. And they work because of chemistry, with some physics thrown in. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. Today’s airbags use a different chemical to produce nitrogen gas: Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. If you’re in a car accident, you want to be sure your airbags protect you. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant.
from www.numerade.com
Most cars have airbags built into the dashboard and steering. Manufacturers use different chemical stews to fill their airbags. If you’re in a car accident, you want to be sure your airbags protect you. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Today’s airbags use a different chemical to produce nitrogen gas: The chemical at the heart of. This week on reactions, we’re. The answer would be found in a fascinating chemical called sodium azide, nan3. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. And they work because of chemistry, with some physics thrown in.
SOLVED The inflation of airbags is due to the breakdown of a single
Chemical Reaction Used In Airbags When this substance is ignited by a spark it releases nitrogen gas which can. Most cars have airbags built into the dashboard and steering. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Guanidinium nitrate, plus a copper nitrate oxidizer. Today’s airbags use a different chemical to produce nitrogen gas: If you’re in a car accident, you want to be sure your airbags protect you. The answer would be found in a fascinating chemical called sodium azide, nan3. This week on reactions, we’re. Did you know that a really fast chemical reaction makes riding in a car safer? When this substance is ignited by a spark it releases nitrogen gas which can. And they work because of chemistry, with some physics thrown in. The chemical at the heart of. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Manufacturers use different chemical stews to fill their airbags. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift.
From exovrvadw.blob.core.windows.net
How Do Airbags Inflate So Quickly at Jane Hill blog Chemical Reaction Used In Airbags Guanidinium nitrate, plus a copper nitrate oxidizer. Manufacturers use different chemical stews to fill their airbags. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. When this substance is. Chemical Reaction Used In Airbags.
From www.gmride.com.my
Supplemental Restraint System Airbags How Does It Work? Guard My Ride Chemical Reaction Used In Airbags Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. When this substance is ignited by a spark it releases nitrogen gas which can. The answer would be found in a fascinating chemical called sodium azide, nan3. This week on reactions, we’re. And they work because of chemistry, with. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVED Sodium azide is the chemical used in airbags to inflate them Chemical Reaction Used In Airbags The answer would be found in a fascinating chemical called sodium azide, nan3. If you’re in a car accident, you want to be sure your airbags protect you. Guanidinium nitrate, plus a copper nitrate oxidizer. And they work because of chemistry, with some physics thrown in. Manufacturers use different chemical stews to fill their airbags. This week on reactions, we’re.. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVED Airbags are present in all new cars being produced today. When Chemical Reaction Used In Airbags Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. Did you know that a really fast chemical reaction makes riding in a car safer? Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. This week on reactions, we’re.. Chemical Reaction Used In Airbags.
From www.chegg.com
Automobile airbags inflate due to the formation of Chemical Reaction Used In Airbags When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. The chemical at the heart of. Manufacturers use different chemical stews to fill their airbags. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. When this substance is. Chemical Reaction Used In Airbags.
From gomechanic.in
The History Behind Airbags In Modern Cars Chemical Reaction Used In Airbags The answer would be found in a fascinating chemical called sodium azide, nan3. The chemical at the heart of. Guanidinium nitrate, plus a copper nitrate oxidizer. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Did you know that a really fast chemical reaction makes riding in a car safer?. Chemical Reaction Used In Airbags.
From www.chegg.com
What mass of sodium azide is required to inflate a Chemical Reaction Used In Airbags When this substance is ignited by a spark it releases nitrogen gas which can. Today’s airbags use a different chemical to produce nitrogen gas: Manufacturers use different chemical stews to fill their airbags. Most cars have airbags built into the dashboard and steering. And they work because of chemistry, with some physics thrown in. Most airbags are inflated when the. Chemical Reaction Used In Airbags.
From www.caranddriver.com
The Physics Of Airbags Chemical Reaction Used In Airbags Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Guanidinium nitrate, plus a copper nitrate oxidizer. When car sensors detect a crash, a chemical reaction is triggered by the. Chemical Reaction Used In Airbags.
From exykgdpcd.blob.core.windows.net
What Chemicals Are Used In Airbags at Brian Clodfelter blog Chemical Reaction Used In Airbags Guanidinium nitrate, plus a copper nitrate oxidizer. When this substance is ignited by a spark it releases nitrogen gas which can. Today’s airbags use a different chemical to produce nitrogen gas: Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. The chemical at the heart of. Manufacturers use. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVED 3. Automobile airbags work by triggering the spontaneous Chemical Reaction Used In Airbags And they work because of chemistry, with some physics thrown in. When this substance is ignited by a spark it releases nitrogen gas which can. Guanidinium nitrate, plus a copper nitrate oxidizer. The answer would be found in a fascinating chemical called sodium azide, nan3. Most airbags are inflated when the inflator unit ignites a pellet of a compound called. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVEDCar safety airbags inflate when the car undergoes a sudden Chemical Reaction Used In Airbags Most cars have airbags built into the dashboard and steering. Manufacturers use different chemical stews to fill their airbags. And they work because of chemistry, with some physics thrown in. When this substance is ignited by a spark it releases nitrogen gas which can. Guanidinium nitrate, plus a copper nitrate oxidizer. If you’re in a car accident, you want to. Chemical Reaction Used In Airbags.
From www.nsta.org
Airbags as RealLife Applications for Science NSTA Chemical Reaction Used In Airbags The chemical at the heart of. Did you know that a really fast chemical reaction makes riding in a car safer? Manufacturers use different chemical stews to fill their airbags. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. Today’s airbags use a different chemical to produce nitrogen. Chemical Reaction Used In Airbags.
From www.chegg.com
Solved The emergency airbags in modern automobiles use a Chemical Reaction Used In Airbags Today’s airbags use a different chemical to produce nitrogen gas: Manufacturers use different chemical stews to fill their airbags. The answer would be found in a fascinating chemical called sodium azide, nan3. This week on reactions, we’re. When this substance is ignited by a spark it releases nitrogen gas which can. Most airbags are inflated when the inflator unit ignites. Chemical Reaction Used In Airbags.
From www.scribd.com
Entropy and Free Energy Day 1 Skeleton Notes PDF Airbag Chemical Chemical Reaction Used In Airbags The answer would be found in a fascinating chemical called sodium azide, nan3. This week on reactions, we’re. When this substance is ignited by a spark it releases nitrogen gas which can. Most cars have airbags built into the dashboard and steering. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3),. Chemical Reaction Used In Airbags.
From www.youtube.com
CHEMICAL REACTION THAT HAPPENS IN CAR AIRBAGS GROUP 3 (EXCELLENCE Chemical Reaction Used In Airbags Most cars have airbags built into the dashboard and steering. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Guanidinium nitrate, plus a copper nitrate oxidizer. When this substance is ignited by a spark it releases nitrogen gas which can. Did you know that a really fast chemical reaction. Chemical Reaction Used In Airbags.
From www.coplancrane.com
Can Car Airbags Cause Chemical Burns? Chicago, Illinois Chemical Reaction Used In Airbags When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. And they work because of chemistry, with some physics thrown in. Today’s airbags use a different chemical to produce nitrogen gas: Did you know that a really fast chemical reaction makes riding in a car safer? When this substance is. Chemical Reaction Used In Airbags.
From www.studocu.com
The Chemistry of Airbags C11209 Questions are being raised as to Chemical Reaction Used In Airbags When this substance is ignited by a spark it releases nitrogen gas which can. Today’s airbags use a different chemical to produce nitrogen gas: If you’re in a car accident, you want to be sure your airbags protect you. Guanidinium nitrate, plus a copper nitrate oxidizer. Air bags are not inflated from some compressed gas source but rather from the. Chemical Reaction Used In Airbags.
From somaap.org
How does airbag deployment work, What is the science behind airbags? Chemical Reaction Used In Airbags Today’s airbags use a different chemical to produce nitrogen gas: Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. When this substance is ignited by a spark it releases nitrogen gas which can. Guanidinium nitrate, plus a copper nitrate oxidizer. Most cars have airbags built into the dashboard and steering.. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVED Automobile airbags inflate due to the formation of nitrogen gas Chemical Reaction Used In Airbags Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. If you’re in a car accident, you want to be sure your airbags protect you. The answer would be found in a fascinating chemical called sodium azide, nan3. Manufacturers use different chemical stews to fill their airbags. And they. Chemical Reaction Used In Airbags.
From slideplayer.com
Intro—Airbags. ppt download Chemical Reaction Used In Airbags Did you know that a really fast chemical reaction makes riding in a car safer? If you’re in a car accident, you want to be sure your airbags protect you. Today’s airbags use a different chemical to produce nitrogen gas: Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Guanidinium. Chemical Reaction Used In Airbags.
From armsingle10.pythonanywhere.com
Glory Airbag Chemical Equation Balanced Equations For Photosynthesis Chemical Reaction Used In Airbags And they work because of chemistry, with some physics thrown in. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Manufacturers use different chemical stews to fill their. Chemical Reaction Used In Airbags.
From slideplayer.com
Collision Theory and Rates of Reactions ppt download Chemical Reaction Used In Airbags And they work because of chemistry, with some physics thrown in. The answer would be found in a fascinating chemical called sodium azide, nan3. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Guanidinium nitrate, plus a copper nitrate oxidizer. If you’re in a car accident, you want to. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVEDThe production of nitrogen gas for automobile airbags takes Chemical Reaction Used In Airbags The chemical at the heart of. This week on reactions, we’re. When this substance is ignited by a spark it releases nitrogen gas which can. And they work because of chemistry, with some physics thrown in. The answer would be found in a fascinating chemical called sodium azide, nan3. Manufacturers use different chemical stews to fill their airbags. Did you. Chemical Reaction Used In Airbags.
From www.youtube.com
The Science Behind Airbags Airbag chemical reaction SUSHMITA SHARMA Chemical Reaction Used In Airbags Guanidinium nitrate, plus a copper nitrate oxidizer. When this substance is ignited by a spark it releases nitrogen gas which can. Did you know that a really fast chemical reaction makes riding in a car safer? This week on reactions, we’re. The answer would be found in a fascinating chemical called sodium azide, nan3. When car sensors detect a crash,. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVEDSodium azide, the explosive chemical used in automobile airbags Chemical Reaction Used In Airbags This week on reactions, we’re. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. And they work because of chemistry, with some physics thrown in. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Today’s airbags use a different. Chemical Reaction Used In Airbags.
From www.youtube.com
Chemical Reaction in a Car Airbag YouTube Chemical Reaction Used In Airbags Most cars have airbags built into the dashboard and steering. Guanidinium nitrate, plus a copper nitrate oxidizer. When this substance is ignited by a spark it releases nitrogen gas which can. If you’re in a car accident, you want to be sure your airbags protect you. The answer would be found in a fascinating chemical called sodium azide, nan3. When. Chemical Reaction Used In Airbags.
From blog.olx.com.pk
How Airbags Work and Save Lives Chemical Reaction Used In Airbags And they work because of chemistry, with some physics thrown in. This week on reactions, we’re. The answer would be found in a fascinating chemical called sodium azide, nan3. Today’s airbags use a different chemical to produce nitrogen gas: When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Did. Chemical Reaction Used In Airbags.
From www.chegg.com
Solved NEED EXTREME HELP!! When designing an airbag... how Chemical Reaction Used In Airbags When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift.. Chemical Reaction Used In Airbags.
From www.numerade.com
Airbags fill with nitrogen gas from a series of reactions beginning Chemical Reaction Used In Airbags When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. The chemical at the heart of. This week on reactions, we’re. Did you know that a really fast chemical reaction makes riding in a car safer? If you’re in a car accident, you want to be sure your airbags protect. Chemical Reaction Used In Airbags.
From www.haikudeck.com
AIRBAGS by Brandon Romano Chemical Reaction Used In Airbags And they work because of chemistry, with some physics thrown in. Manufacturers use different chemical stews to fill their airbags. Did you know that a really fast chemical reaction makes riding in a car safer? The answer would be found in a fascinating chemical called sodium azide, nan3. The chemical at the heart of. Today’s airbags use a different chemical. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVEDSodium azide, an explosive chemical used in automobile airbags Chemical Reaction Used In Airbags Most cars have airbags built into the dashboard and steering. Manufacturers use different chemical stews to fill their airbags. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. The chemical at the heart of. When this substance is ignited by a spark it releases nitrogen gas which can. The answer. Chemical Reaction Used In Airbags.
From www.numerade.com
SOLVED The inflation of airbags is due to the breakdown of a single Chemical Reaction Used In Airbags If you’re in a car accident, you want to be sure your airbags protect you. Most cars have airbags built into the dashboard and steering. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Today’s airbags use a different chemical to produce nitrogen gas: Manufacturers use different chemical stews. Chemical Reaction Used In Airbags.
From innovationdiscoveries.space
What is Airbag? How it works during an accident? Chemical Reaction Used In Airbags This week on reactions, we’re. The chemical at the heart of. Manufacturers use different chemical stews to fill their airbags. Did you know that a really fast chemical reaction makes riding in a car safer? Most airbags are inflated when the inflator unit ignites a pellet of a compound called sodium azide (nan3), kickstarting a swift. When this substance is. Chemical Reaction Used In Airbags.
From www.theatlantic.com
Should Takata Airbags Be Using Ammonium Nitrate as Propellant? The Chemical Reaction Used In Airbags Manufacturers use different chemical stews to fill their airbags. And they work because of chemistry, with some physics thrown in. When car sensors detect a crash, a chemical reaction is triggered by the ignition of a solid hunk of propellant. Air bags are not inflated from some compressed gas source but rather from the products of a chemical reaction. The. Chemical Reaction Used In Airbags.
From www.youtube.com
Airbag The Life saving Chemistry YouTube Chemical Reaction Used In Airbags Manufacturers use different chemical stews to fill their airbags. When this substance is ignited by a spark it releases nitrogen gas which can. Guanidinium nitrate, plus a copper nitrate oxidizer. If you’re in a car accident, you want to be sure your airbags protect you. When car sensors detect a crash, a chemical reaction is triggered by the ignition of. Chemical Reaction Used In Airbags.