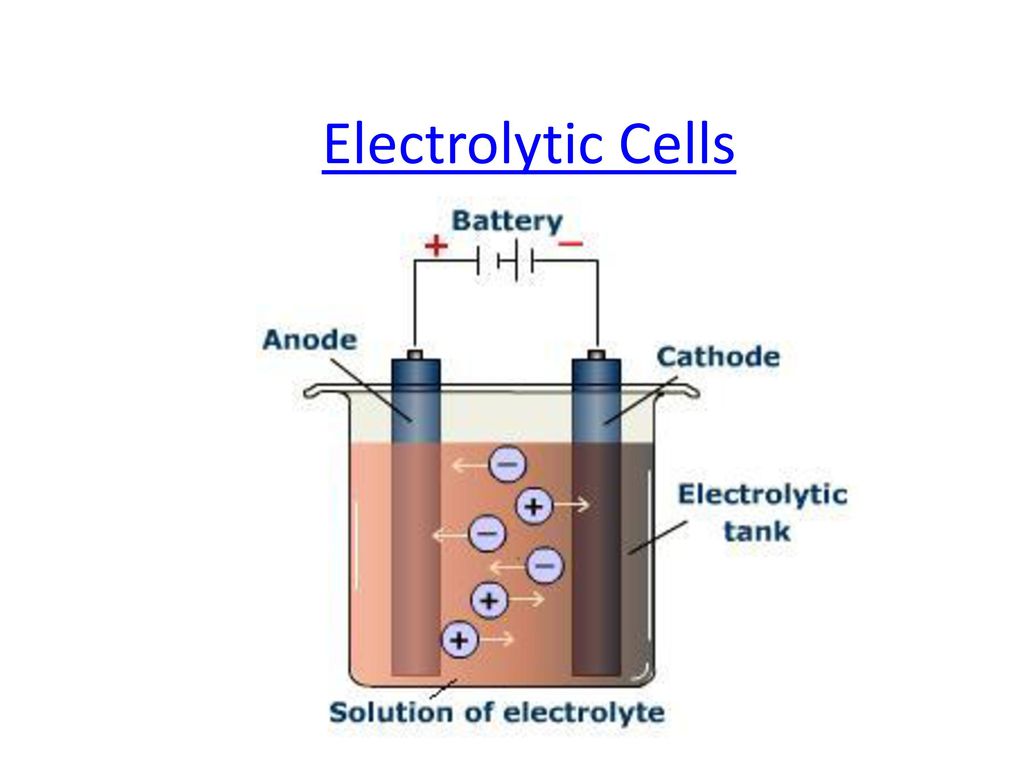
Difference Between Galvanic Cell And Electrochemical Cells . A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell.
from dxoufitvg.blob.core.windows.net
Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy.
What's The Difference Between Galvanic And Electrochemical Cells at
Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical.
From nanoren.blogspot.com
NANOTECHNOLOGY, RENEWABLE ENERGY & CATALYSIS LABORATORY (NRCL Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate. Difference Between Galvanic Cell And Electrochemical Cells.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main difference. Difference Between Galvanic Cell And Electrochemical Cells.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells,. Difference Between Galvanic Cell And Electrochemical Cells.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell. Difference Between Galvanic Cell And Electrochemical Cells.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above. Difference Between Galvanic Cell And Electrochemical Cells.
From dxoufitvg.blob.core.windows.net
What's The Difference Between Galvanic And Electrochemical Cells at Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic (voltaic). Difference Between Galvanic Cell And Electrochemical Cells.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE Difference Between Galvanic Cell And Electrochemical Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main difference between. Difference Between Galvanic Cell And Electrochemical Cells.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing. Difference Between Galvanic Cell And Electrochemical Cells.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between a galvanic and electrolytic cell. Difference Between Galvanic Cell And Electrochemical Cells.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above. Difference Between Galvanic Cell And Electrochemical Cells.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at Difference Between Galvanic Cell And Electrochemical Cells From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an. Difference Between Galvanic Cell And Electrochemical Cells.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. Galvanic cells generate. Difference Between Galvanic Cell And Electrochemical Cells.
From wiringdiagramsmoothest.z21.web.core.windows.net
Car Battery Diagram Oxidation Difference Between Galvanic Cell And Electrochemical Cells From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic cell is. Difference Between Galvanic Cell And Electrochemical Cells.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic Difference Between Galvanic Cell And Electrochemical Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic. Difference Between Galvanic Cell And Electrochemical Cells.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Difference Between Galvanic Cell And Electrochemical Cells From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic (voltaic). Difference Between Galvanic Cell And Electrochemical Cells.
From stablediffusionweb.com
electrochemical engineering Prompts Stable Diffusion Online Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current. Difference Between Galvanic Cell And Electrochemical Cells.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main difference. Difference Between Galvanic Cell And Electrochemical Cells.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current. Difference Between Galvanic Cell And Electrochemical Cells.
From classnotes.org.in
Electrochemical Cells Chemistry, Class 12, Electro Chemistry Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an. Difference Between Galvanic Cell And Electrochemical Cells.
From lessonlistwhiteman.z21.web.core.windows.net
Consider The Following Electrochemical Cell Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to. Difference Between Galvanic Cell And Electrochemical Cells.
From classfullsavarins.z21.web.core.windows.net
Ap Chemistry Electrochemistry Multiple Choice Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate. Difference Between Galvanic Cell And Electrochemical Cells.
From www.pinterest.ph
Galvanic Cell and Electrolytic Cell Electrochemical Cells Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. From the above. Difference Between Galvanic Cell And Electrochemical Cells.
From exozfwykx.blob.core.windows.net
Electrochemistry Cells Definition at Carl Moyer blog Difference Between Galvanic Cell And Electrochemical Cells From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an. Difference Between Galvanic Cell And Electrochemical Cells.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to. Difference Between Galvanic Cell And Electrochemical Cells.
From www.youtube.com
Galvanic Cell Vs Electrolytic Cell differences YouTube Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From. Difference Between Galvanic Cell And Electrochemical Cells.
From classfullsavarins.z21.web.core.windows.net
Electrochemistry Ap Chem Review Difference Between Galvanic Cell And Electrochemical Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between. Difference Between Galvanic Cell And Electrochemical Cells.
From saylordotorg.github.io
Describing Electrochemical Cells Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic cell is. Difference Between Galvanic Cell And Electrochemical Cells.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Difference Between Galvanic Cell And Electrochemical Cells From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an. Difference Between Galvanic Cell And Electrochemical Cells.
From wiringdiagramsmoothest.z21.web.core.windows.net
Car Battery Diagram Oxidation Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy. Difference Between Galvanic Cell And Electrochemical Cells.
From www.clutchprep.com
Electrochemical Cells Analytical Chemistry Video Clutch Prep Difference Between Galvanic Cell And Electrochemical Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. Galvanic cells generate electrical energy. Difference Between Galvanic Cell And Electrochemical Cells.
From guidemanualhilar.z21.web.core.windows.net
Draw A Labelled Diagram Of Electrolytic Cell Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main difference between a galvanic and electrolytic cell. Difference Between Galvanic Cell And Electrochemical Cells.
From dxoufitvg.blob.core.windows.net
What's The Difference Between Galvanic And Electrochemical Cells at Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the. Difference Between Galvanic Cell And Electrochemical Cells.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Difference Between Galvanic Cell And Electrochemical Cells A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Galvanic cells generate electrical energy. Difference Between Galvanic Cell And Electrochemical Cells.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate. Difference Between Galvanic Cell And Electrochemical Cells.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Difference Between Galvanic Cell And Electrochemical Cells The main difference between a galvanic and electrolytic cell is that a galvanic cell converts chemical energy into electrical energy whereas an electrolytic cell. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to. Difference Between Galvanic Cell And Electrochemical Cells.