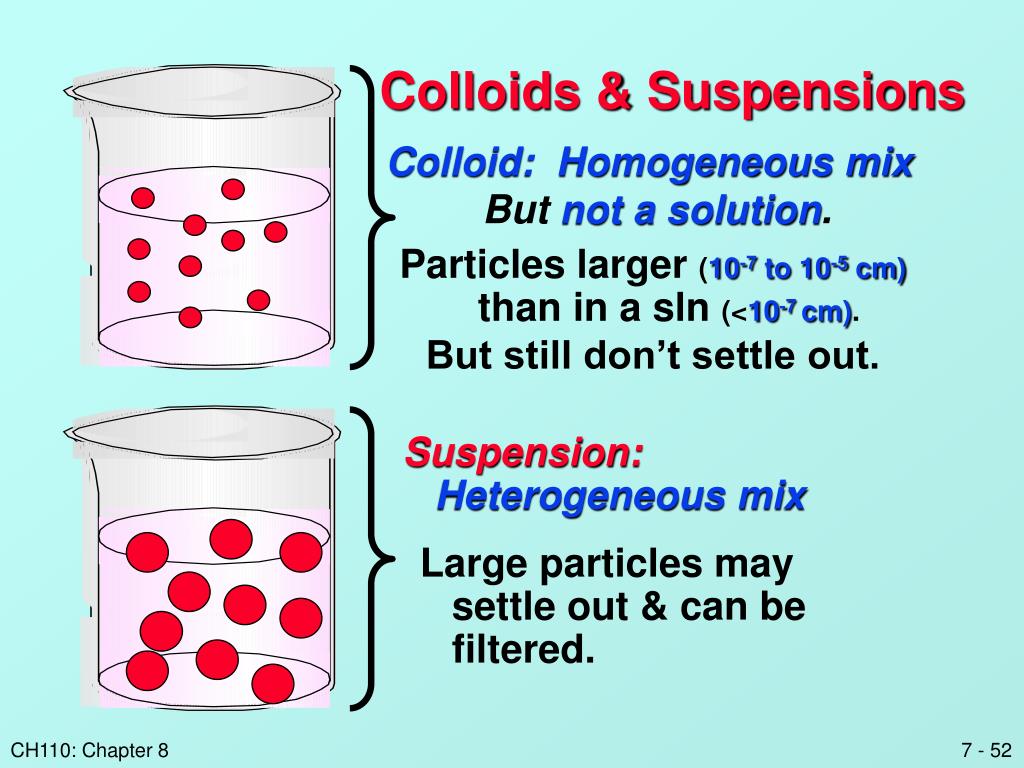
Solution Colloid And Suspension Examples . Suspensions and colloids are two common types of mixtures whose. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is easily visible to the naked eye. To distinguish between true solutions and solutions with aggregate particles. See how it differs from a solution or suspension. A colloid is an intermediate between solution and suspension. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Get the definition and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Learn what a colloid is in chemistry. Compare colloids, suspensions, solutions, and find their differences. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. It has particles with sizes between 2 and 1000 nanometers.
from loezijvab.blob.core.windows.net
Learn what a colloid is in chemistry. It has particles with sizes between 2 and 1000 nanometers. See how it differs from a solution or suspension. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Suspensions and colloids are two common types of mixtures whose. Compare colloids, suspensions, solutions, and find their differences. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloid is an intermediate between solution and suspension. Get the definition and colloid examples. A colloid is easily visible to the naked eye.
Colloid Solution Definition at Gary Reese blog
Solution Colloid And Suspension Examples A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Understand particles in a solution, and explore solution, suspension, and colloid examples. To distinguish between true solutions and solutions with aggregate particles. It has particles with sizes between 2 and 1000 nanometers. Compare colloids, suspensions, solutions, and find their differences. Learn what a colloid is in chemistry. A colloid is easily visible to the naked eye. A colloid is an intermediate between solution and suspension. Suspensions and colloids are two common types of mixtures whose. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. See how it differs from a solution or suspension. Get the definition and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension.
From manualwiringloosebox.z14.web.core.windows.net
Homogeneous Mixture With Dissolved Particles Solution Colloid And Suspension Examples Get the definition and colloid examples. A colloid is an intermediate between solution and suspension. It has particles with sizes between 2 and 1000 nanometers. See how it differs from a solution or suspension. Compare colloids, suspensions, solutions, and find their differences. A colloid is easily visible to the naked eye. Suspensions and colloids differ from solution in that a. Solution Colloid And Suspension Examples.
From loezijvab.blob.core.windows.net
Colloid Solution Definition at Gary Reese blog Solution Colloid And Suspension Examples It has particles with sizes between 2 and 1000 nanometers. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloid is easily visible to the naked eye. Suspensions and colloids are two common types of mixtures whose. A colloid is an intermediate between solution and suspension.. Solution Colloid And Suspension Examples.
From www.youtube.com
Chemistry 9.4 Solutions, Colloids and Suspensions YouTube Solution Colloid And Suspension Examples Compare colloids, suspensions, solutions, and find their differences. To distinguish between true solutions and solutions with aggregate particles. See how it differs from a solution or suspension. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Get the definition and colloid examples. A colloid is a heterogeneous mixture in which the. Solution Colloid And Suspension Examples.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Solution Colloid And Suspension Examples Learn what a colloid is in chemistry. To distinguish between true solutions and solutions with aggregate particles. A colloid is an intermediate between solution and suspension. A colloid is easily visible to the naked eye. It has particles with sizes between 2 and 1000 nanometers. Suspensions and colloids are two common types of mixtures whose. In this chapter, we will. Solution Colloid And Suspension Examples.
From www.slideshare.net
Solutions, suspensions, and colloids Solution Colloid And Suspension Examples Compare colloids, suspensions, solutions, and find their differences. A colloid is an intermediate between solution and suspension. A colloid is easily visible to the naked eye. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It has particles with sizes between 2 and 1000 nanometers. Suspensions. Solution Colloid And Suspension Examples.
From eduardosspeedo.blob.core.windows.net
Suspension Examples Food at eduardosspeedo blog Solution Colloid And Suspension Examples A colloid is an intermediate between solution and suspension. Compare colloids, suspensions, solutions, and find their differences. A colloid is easily visible to the naked eye. Suspensions and colloids are two common types of mixtures whose. Get the definition and colloid examples. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution. Solution Colloid And Suspension Examples.
From captionsfeaturenl.blogspot.com
what is the difference between a solution and a suspension Captions Solution Colloid And Suspension Examples It has particles with sizes between 2 and 1000 nanometers. To distinguish between true solutions and solutions with aggregate particles. A colloid is easily visible to the naked eye. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In this chapter, we will consider the nature. Solution Colloid And Suspension Examples.
From www.youtube.com
Solutions, Suspensions, and Colloids YouTube Solution Colloid And Suspension Examples A colloid is an intermediate between solution and suspension. Get the definition and colloid examples. Learn what a colloid is in chemistry. To distinguish between true solutions and solutions with aggregate particles. See how it differs from a solution or suspension. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is a heterogeneous mixture in. Solution Colloid And Suspension Examples.
From chemistry.about.com
Solutions, Suspensions, Colloids, and Dispersions Solution Colloid And Suspension Examples In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. It has particles with sizes between 2 and 1000 nanometers. Suspensions and colloids are two common types of mixtures whose. A colloid is easily visible to the naked eye. Compare colloids, suspensions, solutions, and find their differences. Suspensions. Solution Colloid And Suspension Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Solution Colloid And Suspension Examples Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids are two common types of mixtures whose. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. See how it differs from a solution or suspension. Understand particles in a solution, and explore solution, suspension, and colloid. Solution Colloid And Suspension Examples.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets Solution Colloid And Suspension Examples A colloid is an intermediate between solution and suspension. See how it differs from a solution or suspension. To distinguish between true solutions and solutions with aggregate particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in. Solution Colloid And Suspension Examples.
From pt.slideshare.net
Solutions, suspensions, and colloids Solution Colloid And Suspension Examples It has particles with sizes between 2 and 1000 nanometers. Compare colloids, suspensions, solutions, and find their differences. Learn what a colloid is in chemistry. Understand particles in a solution, and explore solution, suspension, and colloid examples. Suspensions and colloids are two common types of mixtures whose. Get the definition and colloid examples. Suspensions and colloids differ from solution in. Solution Colloid And Suspension Examples.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Solution Colloid And Suspension Examples Learn what a colloid is in chemistry. To distinguish between true solutions and solutions with aggregate particles. Get the definition and colloid examples. Suspensions and colloids are two common types of mixtures whose. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Compare colloids, suspensions, solutions, and. Solution Colloid And Suspension Examples.
From www.studypage.in
True Solution, Colloidal Solution and Suspension Study Page Solution Colloid And Suspension Examples To distinguish between true solutions and solutions with aggregate particles. Learn what a colloid is in chemistry. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is easily visible to the naked eye. It has particles with sizes between 2 and 1000 nanometers. Compare colloids, suspensions, solutions, and find. Solution Colloid And Suspension Examples.
From pt.slideshare.net
Solutions, suspensions, and colloids Solution Colloid And Suspension Examples Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is an intermediate between solution and suspension. Understand particles in a solution, and explore solution, suspension, and colloid examples. It has particles with sizes between 2 and 1000 nanometers. Suspensions and colloids. Solution Colloid And Suspension Examples.
From www.youtube.com
Solution Suspension Colloid YouTube Solution Colloid And Suspension Examples In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Understand particles in a solution, and explore solution, suspension, and colloid examples. Get the definition and colloid examples. Suspensions and colloids are two common types of mixtures whose. Suspensions and colloids differ from solution in that a solution. Solution Colloid And Suspension Examples.
From giorhfjci.blob.core.windows.net
Colloid Solution Pronunciation at Jodi Bowman blog Solution Colloid And Suspension Examples A colloid is an intermediate between solution and suspension. A colloid is easily visible to the naked eye. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Understand particles in a solution, and explore solution, suspension, and colloid examples. Get the definition and colloid examples. It has. Solution Colloid And Suspension Examples.
From jeopardylabs.com
Properties of Matter Jeopardy MMS Jeopardy Template Solution Colloid And Suspension Examples In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. A colloid is an intermediate between solution and suspension. Learn what a colloid is in chemistry. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Get the definition and. Solution Colloid And Suspension Examples.
From www.gauthmath.com
Solved Check all examples that are homogeneous mixtures. Select all Solution Colloid And Suspension Examples Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is easily visible to the naked eye. See how it differs from a solution or suspension. Learn what a colloid is in chemistry. A colloid is an intermediate between solution and suspension.. Solution Colloid And Suspension Examples.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Solution Colloid And Suspension Examples To distinguish between true solutions and solutions with aggregate particles. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Learn what a colloid is in chemistry. Get the definition and colloid examples. A colloid is easily visible to the naked eye. Suspensions and colloids differ from solution. Solution Colloid And Suspension Examples.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Solution Colloid And Suspension Examples Suspensions and colloids are two common types of mixtures whose. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Learn what a colloid is in chemistry. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid. Solution Colloid And Suspension Examples.
From www.doovi.com
Solution, Suspension and Colloid Doovi Solution Colloid And Suspension Examples See how it differs from a solution or suspension. Get the definition and colloid examples. It has particles with sizes between 2 and 1000 nanometers. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Suspensions and colloids differ from solution in that a solution is homogeneous, contains. Solution Colloid And Suspension Examples.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Solution Colloid And Suspension Examples It has particles with sizes between 2 and 1000 nanometers. Compare colloids, suspensions, solutions, and find their differences. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is easily visible to the naked eye. Learn what a colloid is in chemistry. To distinguish between true solutions and solutions with aggregate particles. A colloid is a. Solution Colloid And Suspension Examples.
From studylib.net
Solutions, Colloids, Suspension Solution Colloid And Suspension Examples Get the definition and colloid examples. A colloid is an intermediate between solution and suspension. See how it differs from a solution or suspension. To distinguish between true solutions and solutions with aggregate particles. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Suspensions and colloids are two common types of. Solution Colloid And Suspension Examples.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Solution Colloid And Suspension Examples It has particles with sizes between 2 and 1000 nanometers. A colloid is an intermediate between solution and suspension. See how it differs from a solution or suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Suspensions and colloids differ from solution in that a. Solution Colloid And Suspension Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Solution Colloid And Suspension Examples Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is an intermediate between solution and suspension. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Get the definition and colloid examples. A colloid is easily visible to the naked eye. Compare colloids, suspensions, solutions, and find. Solution Colloid And Suspension Examples.
From printableadeste.z14.web.core.windows.net
Homogeneous Vs Heterogeneous Chemistry Solution Colloid And Suspension Examples Understand particles in a solution, and explore solution, suspension, and colloid examples. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Suspensions and colloids are two common types of mixtures whose. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form. Solution Colloid And Suspension Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Solution Colloid And Suspension Examples See how it differs from a solution or suspension. Suspensions and colloids are two common types of mixtures whose. Understand particles in a solution, and explore solution, suspension, and colloid examples. It has particles with sizes between 2 and 1000 nanometers. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Solution Colloid And Suspension Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Solution Colloid And Suspension Examples A colloid is an intermediate between solution and suspension. Learn what a colloid is in chemistry. See how it differs from a solution or suspension. To distinguish between true solutions and solutions with aggregate particles. Get the definition and colloid examples. Suspensions and colloids are two common types of mixtures whose. Understand particles in a solution, and explore solution, suspension,. Solution Colloid And Suspension Examples.
From id.pinterest.com
Solutions Colloids and Suspensions Worksheet tomasino S Class Solution Colloid And Suspension Examples Get the definition and colloid examples. Compare colloids, suspensions, solutions, and find their differences. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A colloid is easily visible to the naked eye. It has. Solution Colloid And Suspension Examples.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Solution Colloid And Suspension Examples Learn what a colloid is in chemistry. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It has particles with sizes between 2 and 1000 nanometers. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Suspensions and. Solution Colloid And Suspension Examples.
From www.flexiprep.com
Colloids Properties of True Solution, Colloidal Solution and Solution Colloid And Suspension Examples A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It has particles with sizes between 2 and 1000 nanometers. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what. Suspensions and colloids differ from. Solution Colloid And Suspension Examples.