Is Acetone More Polar Than Ethanol . 5 to 30% is more. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Very easy to remove on a rotary evaporator. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Acetone and ethanol differ in their physical properties. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Common solvents arranged from the least polar to the most polar. It is true that acetone is less polar than ethanol. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Ethanol is the more polar solvent compared to acetone.
from www.numerade.com
However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Acetone and ethanol differ in their physical properties. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. 5 to 30% is more. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Very easy to remove on a rotary evaporator. Ethanol is the more polar solvent compared to acetone. Common solvents arranged from the least polar to the most polar. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol.
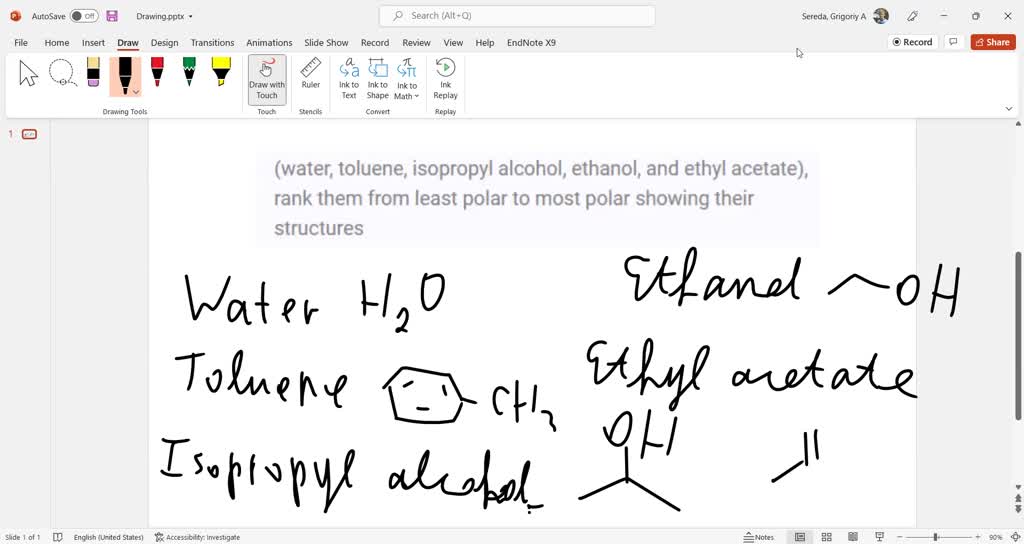
SOLVED Rank hexane, water, ethanol, acetone, and ethyl acetate from
Is Acetone More Polar Than Ethanol Common solvents arranged from the least polar to the most polar. Ethanol is the more polar solvent compared to acetone. 5 to 30% is more. It is true that acetone is less polar than ethanol. Very easy to remove on a rotary evaporator. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Common solvents arranged from the least polar to the most polar. Acetone and ethanol differ in their physical properties.
From www.numerade.com
SOLVED pulal Draw the structures of ethanol, acetone, toluene, hexane Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Ethanol is the more polar solvent compared to acetone. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. 5 to 30% is more. It is true that acetone is less polar than ethanol. Water. Is Acetone More Polar Than Ethanol.
From www.vedantu.com
The most reactive of the following is A Acetone B Benzophenone class 11 Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Very easy to remove on a rotary evaporator. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Water. Is Acetone More Polar Than Ethanol.
From www.numerade.com
SOLVED Rank hexane, water, ethanol, acetone, and ethyl acetate from Is Acetone More Polar Than Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. It is true that acetone is less polar than ethanol. Ethanol is the more polar solvent compared to acetone.. Is Acetone More Polar Than Ethanol.
From chemistry.stackexchange.com
organic chemistry Why bond energy of acetone is more though it is Is Acetone More Polar Than Ethanol It is true that acetone is less polar than ethanol. Common solvents arranged from the least polar to the most polar. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Very easy to remove on a rotary evaporator. Acetone and ethanol differ in their physical properties. However, acetone is still considered a polar aprotic solvent, despite. Is Acetone More Polar Than Ethanol.
From www.chegg.com
Solved 1. A mixture of acetone and ethanol (acetone is more Is Acetone More Polar Than Ethanol Acetone and ethanol differ in their physical properties. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Ethanol is the more polar solvent compared to acetone. 5 to 30% is more. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Common solvents arranged from the least polar to the. Is Acetone More Polar Than Ethanol.
From www.youtube.com
Is C3H6O (Acetone) Polar or NonPolar? YouTube Is Acetone More Polar Than Ethanol It is true that acetone is less polar than ethanol. Ethanol is the more polar solvent compared to acetone. Very easy to remove on a rotary evaporator. Acetone and ethanol differ in their physical properties. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. I thought the dipole moment is. Is Acetone More Polar Than Ethanol.
From www.chegg.com
Solved Common polar solvents Water (H20) Acetone (CH3COCH3) Is Acetone More Polar Than Ethanol Ethanol is the more polar solvent compared to acetone. Acetone and ethanol differ in their physical properties. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine. Is Acetone More Polar Than Ethanol.
From www.coursehero.com
chemistry. Ethanol is more polar than ethane O True O False Submit Is Acetone More Polar Than Ethanol However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Very easy to remove on a. Is Acetone More Polar Than Ethanol.
From www.doubtnut.com
How is acetone obtained from ethanol? Or How is acetic acid converte Is Acetone More Polar Than Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. 5 to 30% is more. Common solvents arranged from the least polar to the most polar. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline. Is Acetone More Polar Than Ethanol.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone More Polar Than Ethanol Acetone and ethanol differ in their physical properties. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. 5 to 30% is. Is Acetone More Polar Than Ethanol.
From www.researchgate.net
Possible reaction route between acetone and ethanol (Onyestyák et Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Very easy to remove on a rotary evaporator. It is true that acetone is less polar than ethanol. However, acetone is still considered a polar aprotic solvent, despite the fact that it is. Is Acetone More Polar Than Ethanol.
From www.numerade.com
SOLVED Arrange the following solvents by polarity (least to most polar Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. 5 to 30% is more. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Is Acetone More Polar Than Ethanol.
From mungfali.com
Acetone Polar Or Nonpolar Is Acetone More Polar Than Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone and ethanol differ in their physical properties. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Is Acetone More Polar Than Ethanol.
From www.youtube.com
Ethanol to acetone Acetone to ethanol Chemical conversion easy Is Acetone More Polar Than Ethanol I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Ethanol is the more polar solvent compared to acetone. It is true that acetone is less polar than ethanol. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Acetone and ethanol differ in their physical properties. Water acetic acid ethyleneglycol. Is Acetone More Polar Than Ethanol.
From www.chegg.com
Solved Based on the structures provide for ethanol, water, Is Acetone More Polar Than Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone and ethanol differ in their physical properties. 5 to 30% is more. Common solvents arranged from the least polar to the most polar. Ethanol is the more polar solvent compared to acetone. Very easy to remove on a rotary evaporator.. Is Acetone More Polar Than Ethanol.
From nationsre.com
Polarity Acetonitrile Water Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Common solvents arranged from the least polar to the most polar. It is true that acetone is less polar than ethanol. Water acetic acid ethyleneglycol. Is Acetone More Polar Than Ethanol.
From mavink.com
Polarity Chart Of Solvents Is Acetone More Polar Than Ethanol It is true that acetone is less polar than ethanol. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Acetone and ethanol differ in their physical properties. However, acetone is. Is Acetone More Polar Than Ethanol.
From www.youtube.com
a mixture of ethanol and acetone shows positive deviation YouTube Is Acetone More Polar Than Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. 5 to 30% is more. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Ethanol is the more polar solvent compared. Is Acetone More Polar Than Ethanol.
From julianajoysgraves.blogspot.com
Acetone Polar or Nonpolar Solvent JulianajoysGraves Is Acetone More Polar Than Ethanol Very easy to remove on a rotary evaporator. It is true that acetone is less polar than ethanol. 5 to 30% is more. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Ethanol is. Is Acetone More Polar Than Ethanol.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone More Polar Than Ethanol Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. However, acetone is still considered a polar aprotic. Is Acetone More Polar Than Ethanol.
From www.chegg.com
Solved Rank The Following Solvents In Order Of Polarity Is Acetone More Polar Than Ethanol Ethanol is the more polar solvent compared to acetone. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone and ethanol. Is Acetone More Polar Than Ethanol.
From www.differencebetween.com
Difference Between Acetone and Isopropyl Alcohol Compare the Is Acetone More Polar Than Ethanol Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. Acetone and ethanol differ in their physical properties. Very easy to remove on a rotary evaporator. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Common solvents arranged from the least polar to the most polar.. Is Acetone More Polar Than Ethanol.
From byjus.com
34. Acetone is treated with excess of ethanol in the presence of Is Acetone More Polar Than Ethanol It is true that acetone is less polar than ethanol. 5 to 30% is more. Acetone and ethanol differ in their physical properties. Very easy to remove on a rotary evaporator. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol.. Is Acetone More Polar Than Ethanol.
From exoxnpydf.blob.core.windows.net
Why Is Acetone More Polar Than Ethyl Acetate at Linda Haley blog Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. It is true that acetone is less polar than ethanol. Common solvents arranged from the least polar to the most polar. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Acetone and ethanol differ in their physical properties. Ethanol is the more. Is Acetone More Polar Than Ethanol.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone More Polar Than Ethanol 5 to 30% is more. It is true that acetone is less polar than ethanol. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Acetone has a lower boiling point of. Is Acetone More Polar Than Ethanol.
From www.youtube.com
Is acetone polar or nonpolar? Will NaCl dissolve in it? YouTube Is Acetone More Polar Than Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. 5 to 30% is more. It is true that acetone is. Is Acetone More Polar Than Ethanol.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Is Acetone More Polar Than Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Acetone and ethanol differ in their physical properties. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. It is true that acetone is less polar than ethanol. 5 to 30% is more. Very. Is Acetone More Polar Than Ethanol.
From www.numerade.com
SOLVED Hzo Hzo Polar or nonpolar? hexane ethanol acetone hexane Polar Is Acetone More Polar Than Ethanol Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. It is true that acetone is less polar than ethanol. Common solvents arranged from the least polar to the most polar. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. 5 to 30% is more. Acetone. Is Acetone More Polar Than Ethanol.
From www.youtube.com
Ethanol to acetone YouTube Is Acetone More Polar Than Ethanol Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic,. Is Acetone More Polar Than Ethanol.
From www.researchgate.net
Polarizability spectra for water, acetone, and ethanol. Each of the Is Acetone More Polar Than Ethanol However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Acetone and ethanol differ in their physical properties. I thought the. Is Acetone More Polar Than Ethanol.
From magdalenakruwsalazar.blogspot.com
Acetone Polar or Nonpolar Solvent MagdalenakruwSalazar Is Acetone More Polar Than Ethanol Acetone and ethanol differ in their physical properties. Very easy to remove on a rotary evaporator. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. It is true that acetone is less polar than ethanol. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Is Acetone More Polar Than Ethanol.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone More Polar Than Ethanol Acetone and ethanol differ in their physical properties. Ethanol is the more polar solvent compared to acetone. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate dioxane acetone dicholoroethane. 5 to 30% is more. It is true that acetone. Is Acetone More Polar Than Ethanol.
From courses.lumenlearning.com
Properties of Liquids Chemistry Is Acetone More Polar Than Ethanol 5 to 30% is more. Common solvents arranged from the least polar to the most polar. Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Acetone has a lower boiling point of approximately 56 degrees celsius, while ethanol. Acetone and ethanol differ in their physical properties. It is true that acetone is less polar than. Is Acetone More Polar Than Ethanol.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Is Acetone More Polar Than Ethanol Acetone and ethanol differ in their physical properties. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Ethanol is the more polar solvent compared to acetone. It is true that acetone is less polar than ethanol. 5 to 30% is more. Water acetic acid ethyleneglycol methanol ethanol. Is Acetone More Polar Than Ethanol.
From www.differencebetween.com
Difference Between Acetone and Ethanol Compare the Difference Between Is Acetone More Polar Than Ethanol However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. 5 to 30% is more. Very easy to remove on a rotary evaporator. Acetone and ethanol differ in their physical properties. I thought the dipole moment is proportional to polarity, and if so, the dipole moment of. Water. Is Acetone More Polar Than Ethanol.