Structure Of Amino Acids At Physiological Ph . We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Identify structural components of an amino acid. Two amino acids have acidic side chains at neutral. Define zwitterion and isoelectric point. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Determine the charge on an amino acid when it is not at the isoelectric point. Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Draw the zwitterion form of a given. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. For these amino acids, the protonated forms predominate at physiological ph (about 7).
from stock.adobe.com
Define zwitterion and isoelectric point. Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Draw the zwitterion form of a given. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Determine the charge on an amino acid when it is not at the isoelectric point. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). For these amino acids, the protonated forms predominate at physiological ph (about 7). We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,.
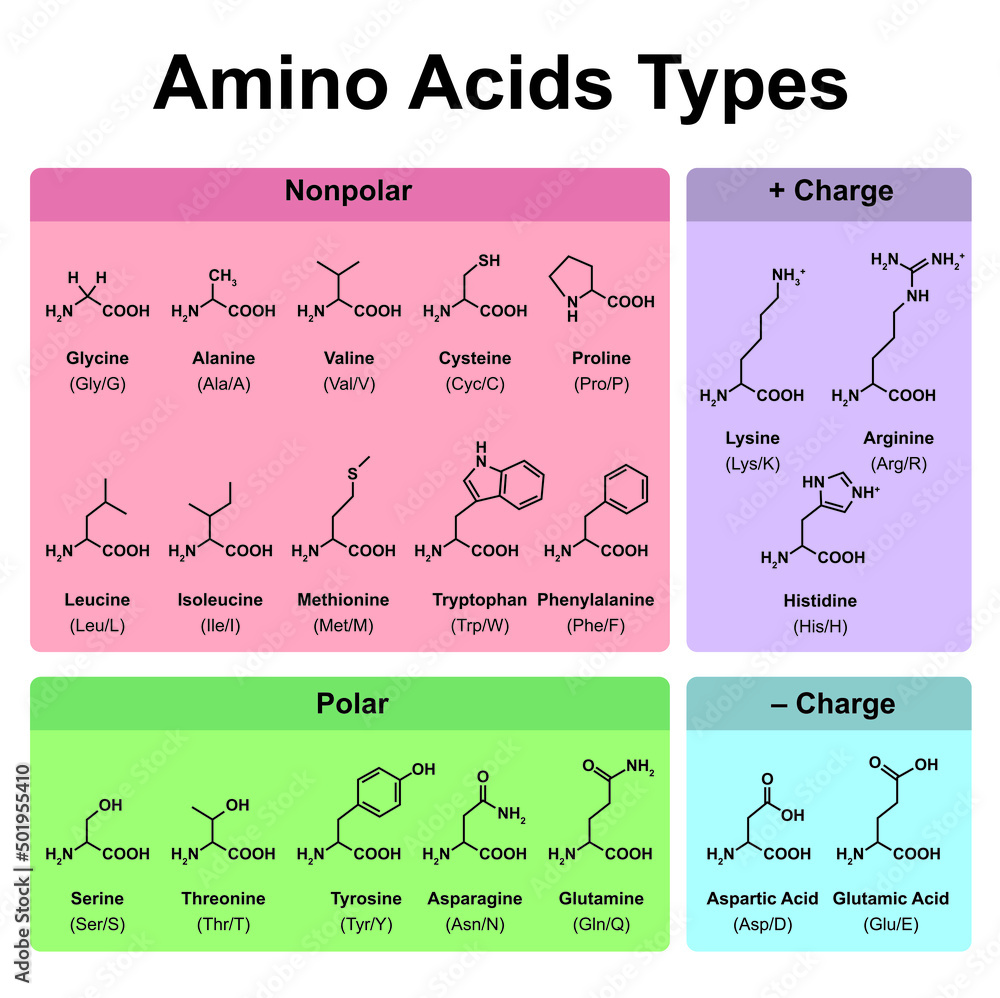
Amino Acids Types Table. Showing The Chemical Structure of Amino Acids
Structure Of Amino Acids At Physiological Ph Identify structural components of an amino acid. Define zwitterion and isoelectric point. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). For these amino acids, the protonated forms predominate at physiological ph (about 7). We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. Identify structural components of an amino acid. Two amino acids have acidic side chains at neutral. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Determine the charge on an amino acid when it is not at the isoelectric point. Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Draw the zwitterion form of a given.
From www.researchgate.net
1 Chemical structure for the 20 amino acids that are found in all Structure Of Amino Acids At Physiological Ph Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. For these amino acids, the protonated forms predominate at physiological ph (about 7). Identify structural components of. Structure Of Amino Acids At Physiological Ph.
From www.rapidnovor.com
Structure of an Amino Acid Rapid Novor Structure Of Amino Acids At Physiological Ph For these amino acids, the protonated forms predominate at physiological ph (about 7). Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino. Structure Of Amino Acids At Physiological Ph.
From mungfali.com
Amino Acid Fischer Projection Structure Of Amino Acids At Physiological Ph Identify structural components of an amino acid. Two amino acids have acidic side chains at neutral. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Define zwitterion and isoelectric point. We saw in section 20.3 and section 24.5 that. Structure Of Amino Acids At Physiological Ph.
From www.creative-biostructure.com
Amino Acid Properties and Structure Creative Biostructure Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. We saw in section. Structure Of Amino Acids At Physiological Ph.
From www.numerade.com
an amino acid backbone is shown draw the glutamate side chain structure Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Two amino acids have acidic side chains at neutral. Define zwitterion and isoelectric point. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists. Structure Of Amino Acids At Physiological Ph.
From socratic.org
How does pH affect amino acid structure? + Example Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Draw the zwitterion form of a given. Determine the charge on an amino acid when it is not at the isoelectric point. When calculating the pi of an amino acid. Structure Of Amino Acids At Physiological Ph.
From onlinesciencenotes.com
Amino acids General properties and classification Online Science Notes Structure Of Amino Acids At Physiological Ph Identify structural components of an amino acid. Draw the zwitterion form of a given. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists. Structure Of Amino Acids At Physiological Ph.
From www.medschoolcoach.com
Amino Acid Classification MCAT Biochemistry MedSchoolCoach Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. Identify structural components of an amino acid. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a. Structure Of Amino Acids At Physiological Ph.
From basicmedicalkey.com
Structure, Nomenclature, and Properties of Proteins and Amino Acids Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Two amino acids have acidic side chains at neutral. Draw the zwitterion form of a given. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with. Structure Of Amino Acids At Physiological Ph.
From www.youtube.com
69 Structure of basic amino acids at various pH YouTube Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Two amino acids have acidic side chains at neutral. Draw the zwitterion form of a given. For these amino acids, the protonated forms predominate at physiological ph (about 7). We. Structure Of Amino Acids At Physiological Ph.
From aminodotnet.weebly.com
Nature of Amino Acids Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. Draw the zwitterion form of a given. Determine the charge on an amino acid when it is not at the isoelectric point.. Structure Of Amino Acids At Physiological Ph.
From www.chegg.com
Solved Given the following four amino acids at pH = 7.4, Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. For these amino acids, the protonated forms predominate at physiological ph (about 7). Define zwitterion and isoelectric point. We saw in section 20.3 and section 24.5 that a carboxyl group. Structure Of Amino Acids At Physiological Ph.
From www.drnerz.com
Amino Acid Structures at Physiological pH Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. For these amino acids, the protonated forms predominate at physiological ph (about 7). We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. Structure Of Amino Acids At Physiological Ph.
From www.slideserve.com
PPT Proteins Amino Acid Chains PowerPoint Presentation ID7053765 Structure Of Amino Acids At Physiological Ph Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. For these amino acids, the protonated forms predominate at physiological. Structure Of Amino Acids At Physiological Ph.
From www.youtube.com
General Structure of an Amino Acid YouTube Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Determine the charge on an amino acid when it is not at the isoelectric point. Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or. Structure Of Amino Acids At Physiological Ph.
From mungfali.com
Amino Acid Chain Structure Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Identify structural components of an amino acid. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Draw the zwitterion form of. Structure Of Amino Acids At Physiological Ph.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and Structure Of Amino Acids At Physiological Ph Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Determine the charge on an amino acid when it is not at the isoelectric point. Define zwitterion. Structure Of Amino Acids At Physiological Ph.
From www.drnerz.com
Amino Acid Structures at Physiological pH Structure Of Amino Acids At Physiological Ph Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Draw the zwitterion form of a given. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and. Structure Of Amino Acids At Physiological Ph.
From slideplayer.com
PTT103 BIOCHEMISTRY Amino Acids ppt download Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Two amino acids have acidic side chains at neutral. For these amino acids, the protonated forms predominate at physiological. Structure Of Amino Acids At Physiological Ph.
From www.numerade.com
SOLVEDDraw the structure for each of the following amino acids at Structure Of Amino Acids At Physiological Ph When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph. Structure Of Amino Acids At Physiological Ph.
From www.lecturio.com
Basics of Amino Acids Concise Medical Knowledge Structure Of Amino Acids At Physiological Ph We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. Determine the charge on an amino acid when it is not at the isoelectric point. Define zwitterion and isoelectric point. At physiologic ph (approximately 7.4), amino acids. Structure Of Amino Acids At Physiological Ph.
From gbee.edu.vn
Isoelectric Points of Amino Acids (and How To Calculate Them) Gbee Structure Of Amino Acids At Physiological Ph For these amino acids, the protonated forms predominate at physiological ph (about 7). At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as. Structure Of Amino Acids At Physiological Ph.
From www.youtube.com
pH Effects on Amino Acid Structures YouTube Structure Of Amino Acids At Physiological Ph We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph. Structure Of Amino Acids At Physiological Ph.
From www.drnerz.com
Amino Acid Structures at Physiological pH Structure Of Amino Acids At Physiological Ph Draw the zwitterion form of a given. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. For these amino acids, the protonated forms predominate at physiological ph (about 7). When calculating the pi of an amino acid that has a titratable group on. Structure Of Amino Acids At Physiological Ph.
From www.researchgate.net
Twenty common amino acids. Download Scientific Diagram Structure Of Amino Acids At Physiological Ph Determine the charge on an amino acid when it is not at the isoelectric point. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. Classify an amino acid as being acidic, basic or neutral, given its. Structure Of Amino Acids At Physiological Ph.
From pinbezy.weebly.com
Positively charged amino acids pinbezy Structure Of Amino Acids At Physiological Ph Draw the zwitterion form of a given. Identify structural components of an amino acid. Two amino acids have acidic side chains at neutral. Determine the charge on an amino acid when it is not at the isoelectric point. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion. Structure Of Amino Acids At Physiological Ph.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Amino Acids Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Determine the charge on an amino acid when it is not at the isoelectric point. At physiologic ph (approximately. Structure Of Amino Acids At Physiological Ph.
From stock.adobe.com
Amino Acids Types Table. Showing The Chemical Structure of Amino Acids Structure Of Amino Acids At Physiological Ph We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. For these amino acids, the protonated forms predominate at physiological ph (about 7). When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful. Structure Of Amino Acids At Physiological Ph.
From www.slideserve.com
PPT Amino acids, peptides, and proteins PowerPoint Presentation, free Structure Of Amino Acids At Physiological Ph Two amino acids have acidic side chains at neutral. Identify structural components of an amino acid. Classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Determine the charge on an amino acid when it is not at the isoelectric point. For these amino acids, the protonated forms predominate at physiological ph (about. Structure Of Amino Acids At Physiological Ph.
From www.creative-biostructure.com
Amino Acid Properties and Structure Creative Biostructure Structure Of Amino Acids At Physiological Ph We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group is protonated and. Identify structural components of an amino acid. Draw the zwitterion form of a given. When calculating the pi of an amino acid that has a titratable group. Structure Of Amino Acids At Physiological Ph.
From www.slideserve.com
PPT Introduction to Amino Acids of Medical Importance PowerPoint Structure Of Amino Acids At Physiological Ph We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3,. Draw the zwitterion form of a given. Identify structural components of an amino acid. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion. Structure Of Amino Acids At Physiological Ph.
From www3.nd.edu
Zwitterionic form of the aamino acids that occur vat physiological pH Structure Of Amino Acids At Physiological Ph Determine the charge on an amino acid when it is not at the isoelectric point. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and. Structure Of Amino Acids At Physiological Ph.
From mavink.com
Amino Acid Structure Labeled Structure Of Amino Acids At Physiological Ph Define zwitterion and isoelectric point. Two amino acids have acidic side chains at neutral. At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. Determine the charge on an amino acid when it is not at the isoelectric point. For. Structure Of Amino Acids At Physiological Ph.
From www.slideserve.com
PPT Proteins Structure and Function PowerPoint Presentation, free Structure Of Amino Acids At Physiological Ph For these amino acids, the protonated forms predominate at physiological ph (about 7). Determine the charge on an amino acid when it is not at the isoelectric point. Identify structural components of an amino acid. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of. Structure Of Amino Acids At Physiological Ph.
From microbenotes.com
Amino Acids Properties, Structure, Classification, Functions Structure Of Amino Acids At Physiological Ph At physiologic ph (approximately 7.4), amino acids are present as zwitter ions with the carboxyl group forming the negatively charged carboxylate ion (coo− coo −), and the amino group is. We saw in section 20.3 and section 24.5 that a carboxyl group is deprotonated and exists as the carboxylate anion at a physiological ph of 7.3, while an amino group. Structure Of Amino Acids At Physiological Ph.