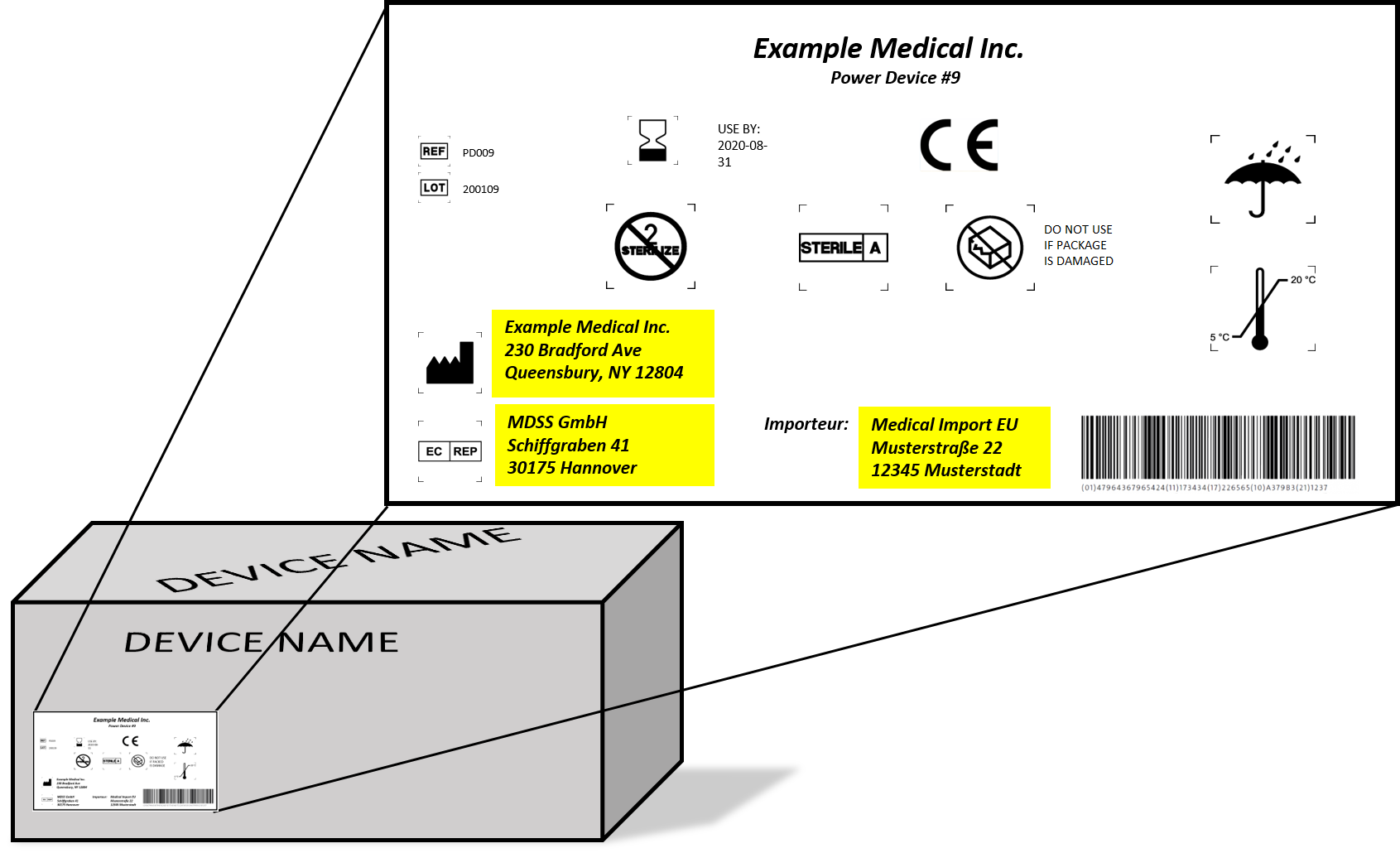
Medical Device Labeling Requirements Mdr . The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745.
from mdssar.com
The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.
MDR and IVDR Services The MDSS Solution!
Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. We will also provide practical strategies to meet.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Medical Device Labeling Requirements Mdr We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places. Medical Device Labeling Requirements Mdr.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr). Medical Device Labeling Requirements Mdr.
From mdlaw.eu
MDR Checklist Labelling & IFU Requirements · MDlaw Information Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used. Medical Device Labeling Requirements Mdr.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. In this blog post, we will delve into the specific labeling requirements outlined in the. Medical Device Labeling Requirements Mdr.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Medical Device Labeling Requirements Mdr In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for. Medical Device Labeling Requirements Mdr.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. In this blog post, we will delve into the specific labeling requirements. Medical Device Labeling Requirements Mdr.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and. Medical Device Labeling Requirements Mdr.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro. Medical Device Labeling Requirements Mdr.
From www.freseniusmedicalcare.com
Medical Device Regulation Fresenius Medical Care Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The eu mdr 2017/745 expands requirements for medical device approvals and places. Medical Device Labeling Requirements Mdr.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products. Medical Device Labeling Requirements Mdr.
From www.mastertrial.com
MDR Requirements for Device Labeling and Implant Card Mastertrial Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design. Medical Device Labeling Requirements Mdr.
From aditi.du.ac.in
MDR Requirements For Device Labeling And Implant Card, 07/24/2023 Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the. Medical Device Labeling Requirements Mdr.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu. Medical Device Labeling Requirements Mdr.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr). Medical Device Labeling Requirements Mdr.
From www.vrogue.co
Guide On Medical Devices Mdivd Ce Marking Mark Europe vrogue.co Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Medical Device Labeling Requirements Mdr.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information. Medical Device Labeling Requirements Mdr.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. We will also provide practical strategies to meet. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various. Medical Device Labeling Requirements Mdr.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used. Medical Device Labeling Requirements Mdr.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of. Medical Device Labeling Requirements Mdr.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design. Medical Device Labeling Requirements Mdr.
From mdssar.com
MDR and IVDR Services The MDSS Solution! Medical Device Labeling Requirements Mdr In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. We will also provide practical strategies to meet. The eu mdr 2017/745 expands requirements for medical device approvals and places additional. Medical Device Labeling Requirements Mdr.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of. Medical Device Labeling Requirements Mdr.
From www.tailoredlabel.com
Medical Device Labeling Impact of MDR TLP Medical Device Labeling Requirements Mdr In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and. Medical Device Labeling Requirements Mdr.
From mavig.com
New Product Labeling due to MDR MAVIG Medical Device Labeling Requirements Mdr In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr). Medical Device Labeling Requirements Mdr.
From medicaldevicehq.com
MDR Article 22 Medical Device HQ Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. We will. Medical Device Labeling Requirements Mdr.
From templates.rjuuc.edu.np
Medical Device Label Template Medical Device Labeling Requirements Mdr We will also provide practical strategies to meet. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances. Medical Device Labeling Requirements Mdr.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements outlined in the eu medical device regulation (mdr) 2017/745. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical. Medical Device Labeling Requirements Mdr.
From gbu-taganskij.ru
Mdr 2017 Guidelines Online Collection gbutaganskij.ru Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. We will also provide practical strategies to meet. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places. Medical Device Labeling Requirements Mdr.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Mdr We will also provide practical strategies to meet. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. In this blog post, we will delve into the specific labeling requirements. Medical Device Labeling Requirements Mdr.
From acf.com.tr
OEM PLM under MDR. Which model you will choose? Medical Device Labeling Requirements Mdr Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. The eu. Medical Device Labeling Requirements Mdr.
From www.presentationeze.com
EU Medical Device Regulation MDR 2017 745PresentationEZE Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro. Medical Device Labeling Requirements Mdr.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Device Labeling Requirements Mdr We will also provide practical strategies to meet. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The medical devices regulation 2017/745/eu (‘mdr’) has. Medical Device Labeling Requirements Mdr.
From gingerproducts.com
Medical device “labelling” language requirements under the EU MDR and Medical Device Labeling Requirements Mdr The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be indicated on the label. We will also provide practical strategies to meet. The medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to be. In this blog post, we will delve into the specific labeling requirements. Medical Device Labeling Requirements Mdr.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labeling Requirements Mdr The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. We will also provide practical strategies to meet. In this blog post, we will delve. Medical Device Labeling Requirements Mdr.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Labeling Requirements Mdr We will also provide practical strategies to meet. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. The eu mdr 2017/745 expands requirements for medical device approvals and places additional restrictions on substances used in the design and manufacture of products to reduce possible. The medical devices regulation 2017/745/eu (‘mdr’) has. Medical Device Labeling Requirements Mdr.