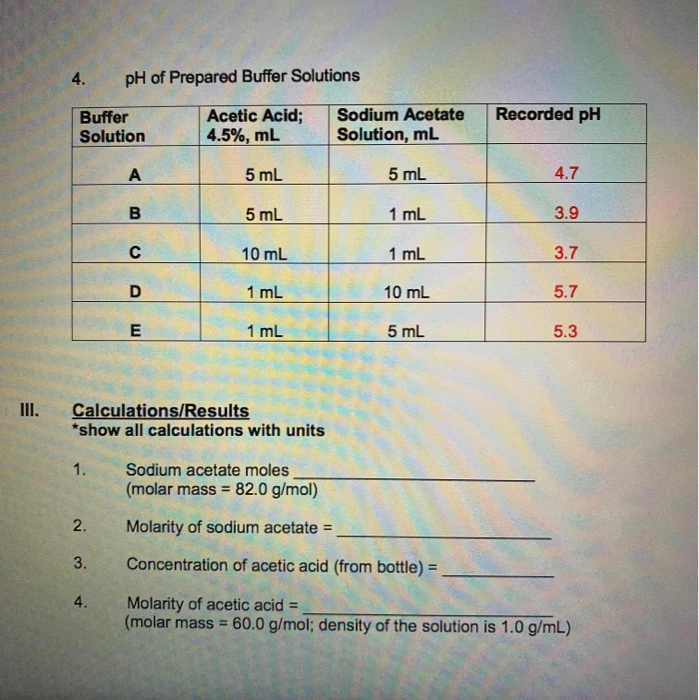
Buffer Solution Lab Questions . The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. A buffer resists a change in ph. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. You are provided with buffer solution. Designing and preparing a buffer. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the.
from www.chegg.com
Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). You are provided with buffer solution. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Designing and preparing a buffer. A buffer resists a change in ph. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most.
Solved Unit IX Buffer Solutions LAB REPORT Name Date
Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. A buffer resists a change in ph. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). You are provided with buffer solution.
From www.chegg.com
Solved Part A Acetic acid/sodium acetate buffers 1. Using a Buffer Solution Lab Questions A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer resists. Buffer Solution Lab Questions.
From www.chegg.com
Solved Buffer Lab Experiment please fill in the table on Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Designing and. Buffer Solution Lab Questions.
From www.studocu.com
GC 4253 L18 Buffer Solutions PRELAB QUESTIONS What would you estimate the pH of hydrochloric Buffer Solution Lab Questions You are provided with buffer solution. A buffer resists a change in ph. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Study with quizlet and memorize flashcards containing terms like. Buffer Solution Lab Questions.
From www.chegg.com
Solved PreLab for Buffer Solutions Lab Name Chem 14 You Buffer Solution Lab Questions Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the. Buffer Solution Lab Questions.
From www.chegg.com
Solved pH and Buffer lab reportI need help with question Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Designing and preparing a buffer. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. A buffer is a solution of a weak acid or base and its salt. Buffer Solution Lab Questions.
From www.chegg.com
Solved Unit IX Buffer Solutions LAB REPORT Name Date Buffer Solution Lab Questions You are provided with buffer solution. Designing and preparing a buffer. A buffer resists a change in ph. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each.. Buffer Solution Lab Questions.
From www.chegg.com
Solved Unit IX Buffer Solutions LAB REPORT Name Date Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Designing and preparing a buffer. You are. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. A buffer resists a change in ph. Designing and preparing a buffer. You are provided with buffer solution. This is a summary practice. Buffer Solution Lab Questions.
From www.chegg.com
Solved Table 4 Buffer Solutions and pH Readings for Beakers Buffer Solution Lab Questions This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. The ability of. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most.. Buffer Solution Lab Questions.
From www.chegg.com
Preparation of Buffer Solutions PRELAB QUESTIONS 1. Buffer Solution Lab Questions Designing and preparing a buffer. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. A buffer resists a change in ph. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Prepare 50 ml from phosphate buffer. Buffer Solution Lab Questions.
From www.studocu.com
Lab 18 exp 2 borax buffer solutions lab Experiment 2Preparing a Buffer Solution with Borax Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Designing and preparing a buffer. A buffer resists a change in ph. You are provided with buffer solution. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). A buffer is a solution of a. Buffer Solution Lab Questions.
From www.chegg.com
Solved Lab Q Preparation and Exploration of a Buffer System Buffer Solution Lab Questions The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. Designing and preparing a buffer. A buffer is a solution of a weak acid or base and its salt. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. You are provided with buffer solution. A buffer is a solution of a weak acid or base and. Buffer Solution Lab Questions.
From www.youtube.com
Buffer solutions 10, lecture 2 YouTube Buffer Solution Lab Questions Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer resists a change in ph. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the. Buffer Solution Lab Questions.
From www.chegg.com
Solved Part 4 Questions 1. a) What is a buffer solution? b) Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. A buffer resists a change in ph. The ability of a buffer solution to resist large changes in ph has a great many. Buffer Solution Lab Questions.
From studylib.net
Chemguide questions BUFFER SOLUTIONS Buffer Solution Lab Questions You are provided with buffer solution. A buffer resists a change in ph. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. This is a summary practice problem set. Buffer Solution Lab Questions.
From studylib.net
experiment 16 buffer solutions Buffer Solution Lab Questions A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). The. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solutions 0 EXPERIMENT 1 Buffer Solution Lab Questions A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. You are provided with buffer solution. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solutions 0 EXPERIMENT 2 Buffer Solution Lab Questions A buffer resists a change in ph. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. You are provided with buffer solution. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Prepare 50 ml from phosphate. Buffer Solution Lab Questions.
From www.chegg.com
Solved Desk Date Hydrolysis of Salts and pH of Buffer Buffer Solution Lab Questions The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Designing and preparing a buffer. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. A buffer is a solution of a weak acid or base and its salt [which is. Buffer Solution Lab Questions.
From www.numerade.com
SOLVED Buffers Observing the Effect of Adding Acid or Base In this experiment you will mix Buffer Solution Lab Questions The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). Study with quizlet and memorize flashcards. Buffer Solution Lab Questions.
From www.chegg.com
Solved Unit IX Buffer Solutions LAB REPORT Name Date Buffer Solution Lab Questions A buffer resists a change in ph. You are provided with buffer solution. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. This is a summary practice problem. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions You are provided with buffer solution. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Designing and preparing a buffer. A buffer resists a change in ph. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). A buffer is a. Buffer Solution Lab Questions.
From studylib.net
Buffer Equilibrium Free Response Questions Buffer Solution Lab Questions Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. You are provided with buffer solution. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. Buffer Solution Lab Questions.
From www.chegg.com
Solved Unit IX Buffer Solutions LAB REPORT Name Date Buffer Solution Lab Questions Designing and preparing a buffer. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. The dissociation of a weak acid in water yields h+ ion and the. Buffer Solution Lab Questions.
From www.numerade.com
SOLVED EXPERIMENT 1 PREPARING A BUFFER Data Sheet Table 3 Sodium Acetate Data Sodium Acetate Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. The ability of a buffer solution to. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. A buffer resists a change in ph. The dissociation of a weak acid in water yields h+ ion and the conjugate base of. Buffer Solution Lab Questions.
From www.studocu.com
Chapter 11 Buffers practice questions ( solutions) 636 pharmaceutics practice questions Buffer Solution Lab Questions A buffer resists a change in ph. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Designing and preparing a buffer. This is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer solution prepared. Prepare 50 ml from phosphate. Buffer Solution Lab Questions.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points earned/18)*15 = total Buffer Solution Lab Questions Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. You are provided with buffer solution. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A buffer resists a change in ph. This is a summary practice. Buffer Solution Lab Questions.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffer Solution Lab Questions A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. A buffer resists a change in ph. Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). Study with quizlet and memorize flashcards containing terms like a buffer solution must contain:, determine whether or not each.. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions Prepare 50 ml from phosphate buffer with concentration 0.25m and ph=7.4, if you know that (pka=7.2). The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. Designing and preparing a. Buffer Solution Lab Questions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Lab Questions A buffer resists a change in ph. A buffer is a solution of a weak acid or base and its salt [which is its conjugate]. The ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. This is a summary practice problem set on buffer solutions aimed to help. Buffer Solution Lab Questions.