Aluminum Oxide Formula Cation And Anion . Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. First, the cation is written before the anion. the formula for an ionic compound follows several conventions. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. aluminum and carbon react to form an ionic compound. This is a neutralisation reaction. Name monoatomic anions and cations. al2o3 + h2so4 → al2 (so4)3 + h2o. Predict which forms an anion, which forms a cation, and the charges of. Because most metals form cations and most. predict the charge of monatomic main group elements based on their group number.
from www.numerade.com
for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. predict the charge of monatomic main group elements based on their group number. al2o3 + h2so4 → al2 (so4)3 + h2o. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. First, the cation is written before the anion. Because most metals form cations and most. the formula for an ionic compound follows several conventions. This is a neutralisation reaction.
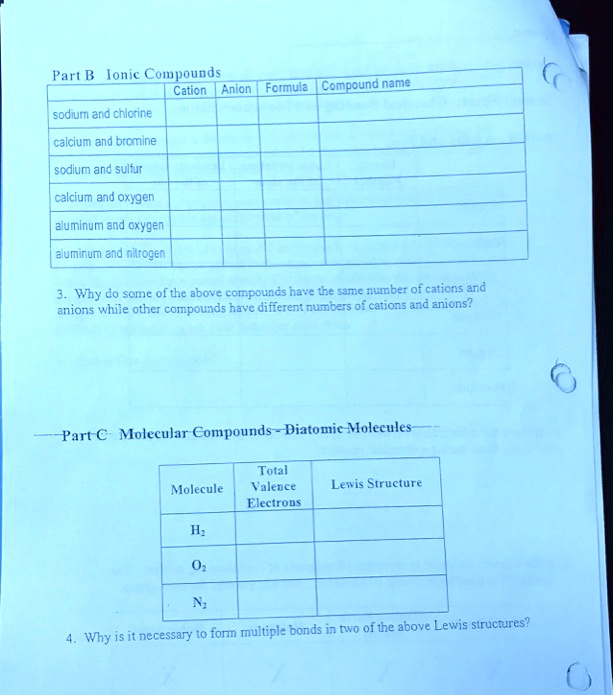
SOLVED Part B Ionic Compounds Anion Formula Compound name Cation
Aluminum Oxide Formula Cation And Anion Predict which forms an anion, which forms a cation, and the charges of. Because most metals form cations and most. predict the charge of monatomic main group elements based on their group number. Predict which forms an anion, which forms a cation, and the charges of. This is a neutralisation reaction. aluminum and carbon react to form an ionic compound. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. al2o3 + h2so4 → al2 (so4)3 + h2o. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Name monoatomic anions and cations. the formula for an ionic compound follows several conventions. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. First, the cation is written before the anion.
From www.numerade.com
SOLVED Table 4 Ionic Compounds and their Names Formula of Cation and Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. al2o3 + h2so4 → al2 (so4)3 + h2o. First, the cation is written before the anion. aluminum and carbon react to form an ionic. Aluminum Oxide Formula Cation And Anion.
From www.numerade.com
SOLVED Ionic Compound Formulas For each of the ionic compounds below Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. Because most metals form cations and most. Predict which forms an anion, which forms a cation, and the charges of. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. for. Aluminum Oxide Formula Cation And Anion.
From www.numerade.com
SOLVED Complete the table below for calculating the molar mass of the Aluminum Oxide Formula Cation And Anion First, the cation is written before the anion. Predict which forms an anion, which forms a cation, and the charges of. This is a neutralisation reaction. al2o3 + h2so4 → al2 (so4)3 + h2o. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every. Aluminum Oxide Formula Cation And Anion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Aluminum Oxide Formula Cation And Anion predict the charge of monatomic main group elements based on their group number. First, the cation is written before the anion. the formula for an ionic compound follows several conventions. Because most metals form cations and most. Name monoatomic anions and cations. This is a neutralisation reaction. Aluminium oxide contains oxide ions, and thus reacts with acids in. Aluminum Oxide Formula Cation And Anion.
From utedzz.blogspot.com
Printable Periodic Table Ionic Charges Periodic Table Timeline Aluminum Oxide Formula Cation And Anion in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. al2o3 + h2so4 → al2 (so4)3 + h2o. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. Aluminium oxide contains. Aluminum Oxide Formula Cation And Anion.
From www.youtube.com
How to Draw the Lewis Dot Structure for Al2O3 Aluminum oxide YouTube Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. predict the charge of monatomic main group elements based on their group number. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. This is a neutralisation reaction. Aluminium oxide contains oxide ions, and thus reacts with acids in. Aluminum Oxide Formula Cation And Anion.
From www.slideshare.net
Ionic bonding binary Aluminum Oxide Formula Cation And Anion for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. This is a neutralisation reaction. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. aluminum and carbon react to form an ionic compound. Because most. Aluminum Oxide Formula Cation And Anion.
From socratic.org
How does ionic radius change across a period? Socratic Aluminum Oxide Formula Cation And Anion in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Predict which forms an anion, which forms a cation, and the charges of. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. aluminum and carbon react to form an. Aluminum Oxide Formula Cation And Anion.
From asyikinkimia7.blogspot.com
BETTER LIVING THROUGH CHEMISTRY Common polyatomic ions Aluminum Oxide Formula Cation And Anion aluminum and carbon react to form an ionic compound. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. This is a neutralisation reaction. Predict which forms an anion, which forms a cation, and the charges of. Name monoatomic anions and. Aluminum Oxide Formula Cation And Anion.
From www.ck12.org
Ionic Compounds CK12 Foundation Aluminum Oxide Formula Cation And Anion for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. al2o3 + h2so4 → al2 (so4)3 + h2o. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide. Aluminum Oxide Formula Cation And Anion.
From www.youtube.com
Lewis Dot Structure for Al2O3 (Aluminum Oxide) YouTube Aluminum Oxide Formula Cation And Anion Predict which forms an anion, which forms a cation, and the charges of. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. al2o3 + h2so4 → al2 (so4)3 + h2o. First, the cation is written before the anion. the formula for an ionic compound follows several conventions. . Aluminum Oxide Formula Cation And Anion.
From ar.inspiredpencil.com
Aluminum Oxide Structure Aluminum Oxide Formula Cation And Anion Name monoatomic anions and cations. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. aluminum and carbon react to form an ionic compound. First, the cation is written before the anion. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains. Aluminum Oxide Formula Cation And Anion.
From www.chegg.com
Solved Date Name Lonic Compound (metal Cation And Nonme... Aluminum Oxide Formula Cation And Anion predict the charge of monatomic main group elements based on their group number. aluminum and carbon react to form an ionic compound. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Name monoatomic anions and cations. for example, the formula for aluminum oxide, al 2 o. Aluminum Oxide Formula Cation And Anion.
From www.slideshare.net
Ch 8 ionic compounds Aluminum Oxide Formula Cation And Anion Name monoatomic anions and cations. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. al2o3 + h2so4 → al2 (so4)3 + h2o. Predict which forms an anion, which forms. Aluminum Oxide Formula Cation And Anion.
From www.slideserve.com
PPT The Nomenclature of Binary Compounds PowerPoint Presentation ID Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. Because most metals form cations and most. al2o3 + h2so4 → al2 (so4)3 + h2o. predict the charge of monatomic main group elements based on their group number. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum. Aluminum Oxide Formula Cation And Anion.
From dxodgqmah.blob.core.windows.net
Chemical Formula For Aluminum And Oxygen at Patrick Moyer blog Aluminum Oxide Formula Cation And Anion in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. Because most metals form cations and most. al2o3 + h2so4 → al2 (so4)3 + h2o.. Aluminum Oxide Formula Cation And Anion.
From www.ilectureonline.com
Aluminum Oxide Formula Cation And Anion for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. Name monoatomic anions and cations. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. al2o3 + h2so4 → al2 (so4)3 + h2o. predict. Aluminum Oxide Formula Cation And Anion.
From www.reddit.com
CHM 116 Lab Help Anion and Cations r/ASU Aluminum Oxide Formula Cation And Anion in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. This is a neutralisation reaction. Because most metals form cations and most. al2o3 + h2so4 → al2 (so4)3 + h2o. First, the cation is written before the anion. for example, the formula for aluminum oxide, al 2 o. Aluminum Oxide Formula Cation And Anion.
From www.numerade.com
SOLVED Part B Ionic Compounds Anion Formula Compound name Cation Aluminum Oxide Formula Cation And Anion This is a neutralisation reaction. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. First, the cation is written before the anion. Because most metals form cations and most. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations,. Aluminum Oxide Formula Cation And Anion.
From chem.libretexts.org
3.1 Ionic Atoms Chemistry LibreTexts Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. al2o3 + h2so4 → al2 (so4)3 + h2o. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Because most metals form cations and most. Name monoatomic anions and cations. Aluminium oxide contains oxide ions, and thus reacts with. Aluminum Oxide Formula Cation And Anion.
From mavink.com
Table Of Cations And Anions Aluminum Oxide Formula Cation And Anion Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Predict which forms an anion, which forms a cation, and the charges of. aluminum and carbon react to form an ionic compound. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains. Aluminum Oxide Formula Cation And Anion.
From saylordotorg.github.io
Naming Ionic Compounds Aluminum Oxide Formula Cation And Anion aluminum and carbon react to form an ionic compound. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. Because most metals form cations and most. Name monoatomic anions and cations. predict the charge of monatomic main group elements based on their group number.. Aluminum Oxide Formula Cation And Anion.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as Aluminum Oxide Formula Cation And Anion predict the charge of monatomic main group elements based on their group number. Predict which forms an anion, which forms a cation, and the charges of. This is a neutralisation reaction. al2o3 + h2so4 → al2 (so4)3 + h2o. Because most metals form cations and most. First, the cation is written before the anion. for example, the. Aluminum Oxide Formula Cation And Anion.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Aluminum Oxide Formula Cation And Anion This is a neutralisation reaction. al2o3 + h2so4 → al2 (so4)3 + h2o. predict the charge of monatomic main group elements based on their group number. Name monoatomic anions and cations. the formula for an ionic compound follows several conventions. Because most metals form cations and most. in the solid state, ionic compounds are in crystal. Aluminum Oxide Formula Cation And Anion.
From slideplayer.com
Chapter 7 Ionic Bonding. ppt download Aluminum Oxide Formula Cation And Anion Predict which forms an anion, which forms a cation, and the charges of. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Name monoatomic anions and. Aluminum Oxide Formula Cation And Anion.
From www.thoughtco.com
The Difference Between a Cation and an Anion Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. First, the cation is written before the anion. aluminum and carbon react to form an ionic compound. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. Name monoatomic anions and cations. Because most metals. Aluminum Oxide Formula Cation And Anion.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Formula For Aluminium Oxide Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. aluminum and carbon react to form an ionic compound. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. This. Aluminum Oxide Formula Cation And Anion.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Aluminum Oxide Formula Cation And Anion predict the charge of monatomic main group elements based on their group number. Predict which forms an anion, which forms a cation, and the charges of. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Because most metals form cations and most. the formula for an ionic. Aluminum Oxide Formula Cation And Anion.
From www.slideshare.net
Ionic bonding binary Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. Predict which forms an anion, which forms a cation, and the charges of. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium. Aluminum Oxide Formula Cation And Anion.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Formula Cation And Anion This is a neutralisation reaction. in the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion. the formula for an ionic compound follows several conventions. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. aluminum and carbon react to. Aluminum Oxide Formula Cation And Anion.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Aluminum Oxide Formula Cation And Anion First, the cation is written before the anion. This is a neutralisation reaction. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+,. Aluminum Oxide Formula Cation And Anion.
From www.youtube.com
Al 3+ Electron Configuration (Aluminum Ion) YouTube Aluminum Oxide Formula Cation And Anion al2o3 + h2so4 → al2 (so4)3 + h2o. Name monoatomic anions and cations. Because most metals form cations and most. This is a neutralisation reaction. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. First, the cation is written before the anion. in the solid state, ionic compounds. Aluminum Oxide Formula Cation And Anion.
From dxobwpkzi.blob.core.windows.net
Bromine Form Anions Or Cations at Frances Parker blog Aluminum Oxide Formula Cation And Anion for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. Predict which forms an anion, which forms a cation, and the charges of. Because most metals form cations and most. Aluminium oxide contains oxide ions, and thus reacts with acids in the. Aluminum Oxide Formula Cation And Anion.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Formula Cation And Anion Because most metals form cations and most. This is a neutralisation reaction. aluminum and carbon react to form an ionic compound. predict the charge of monatomic main group elements based on their group number. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for.. Aluminum Oxide Formula Cation And Anion.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as Aluminum Oxide Formula Cation And Anion the formula for an ionic compound follows several conventions. Predict which forms an anion, which forms a cation, and the charges of. Because most metals form cations and most. for example, the formula for aluminum oxide, al 2 o 3, indicates that this ionic compound contains two aluminum cations, al 3+, for every three oxide anions,. Name monoatomic. Aluminum Oxide Formula Cation And Anion.