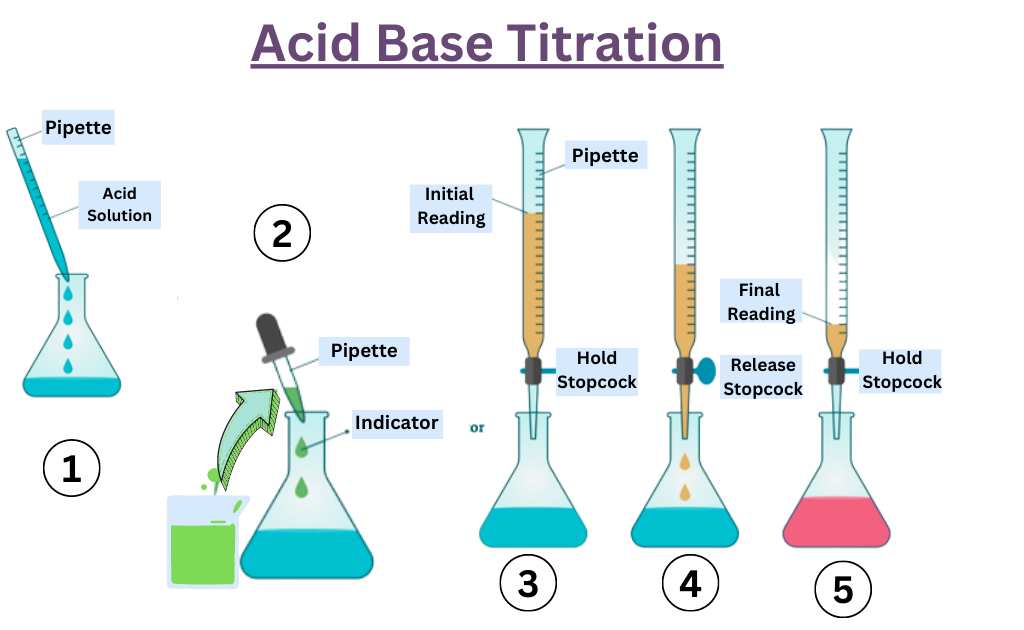
Titration Labelled Diagram . titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The figure below shows two different examples of a strong acid. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. a titration curve is a graphical representation of the ph of a solution during a titration. In each case, you start with 25. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. The end point of a titration is the point at. — there are two basic types of acid base titrations, indicator and potentiometric.
from themasterchemistry.com
the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The figure below shows two different examples of a strong acid. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at. In each case, you start with 25. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. — there are two basic types of acid base titrations, indicator and potentiometric.
Acid Base TitrationWorking Principle, Process, Types And Indicators
Titration Labelled Diagram the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. The end point of a titration is the point at. The figure below shows two different examples of a strong acid. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — there are two basic types of acid base titrations, indicator and potentiometric. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In each case, you start with 25.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Labelled Diagram The figure below shows two different examples of a strong acid. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. — there are two basic types of acid base titrations, indicator and potentiometric. titration involves the gradual addition of a reagent of known. Titration Labelled Diagram.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Labelled Diagram the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. . Titration Labelled Diagram.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Labelled Diagram titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The figure below shows two different examples of a strong acid. — the shape of. Titration Labelled Diagram.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Titration Labelled Diagram It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The figure below shows two different examples of a strong acid. In each case, you start with 25. The end point of a titration is the point at. — the shape of a titration curve, a plot of ph. Titration Labelled Diagram.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Labelled Diagram The end point of a titration is the point at. In each case, you start with 25. a titration curve is a graphical representation of the ph of a solution during a titration. — there are two basic types of acid base titrations, indicator and potentiometric. titration involves the gradual addition of a reagent of known concentration,. Titration Labelled Diagram.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Titration Labelled Diagram — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In each case, you start with 25. — there are two basic types of acid base titrations, indicator. Titration Labelled Diagram.
From www.savemyexams.com
AcidBase Titrations (12.1.3) CIE IGCSE Chemistry Revision Notes 2023 Titration Labelled Diagram The figure below shows two different examples of a strong acid. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. — the shape of a titration curve,. Titration Labelled Diagram.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Labelled Diagram The end point of a titration is the point at. In each case, you start with 25. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — there are two basic types of acid base titrations, indicator and potentiometric. — the shape of. Titration Labelled Diagram.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Labelled Diagram — there are two basic types of acid base titrations, indicator and potentiometric. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. the. Titration Labelled Diagram.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration Labelled Diagram The end point of a titration is the point at. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — there are two basic types of. Titration Labelled Diagram.
From www.vrogue.co
Acid Base Titrations Mcguire Chemistry Libretexts vrogue.co Titration Labelled Diagram In each case, you start with 25. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. the titration curves shown in figure 14.20 illustrate the. Titration Labelled Diagram.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Labelled Diagram a titration curve is a graphical representation of the ph of a solution during a titration. — there are two basic types of acid base titrations, indicator and potentiometric. The end point of a titration is the point at. The figure below shows two different examples of a strong acid. It is based on the neutralization reaction, where. Titration Labelled Diagram.
From mungfali.com
Acid Base Titration Graph Titration Labelled Diagram a titration curve is a graphical representation of the ph of a solution during a titration. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The end point of a titration is the point at. — there are two basic types of acid. Titration Labelled Diagram.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Labelled Diagram In each case, you start with 25. — there are two basic types of acid base titrations, indicator and potentiometric. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — the shape of a titration curve, a plot of ph versus the amount. Titration Labelled Diagram.
From exosvfdmm.blob.core.windows.net
Titration Biology at Pinero blog Titration Labelled Diagram — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In each case, you start with 25. The figure below shows two different examples of a strong acid. . Titration Labelled Diagram.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Labelled Diagram The end point of a titration is the point at. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The figure below shows two different examples. Titration Labelled Diagram.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Labelled Diagram titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a titration curve is a graphical representation of the ph of a solution during a titration. It is based on the neutralization reaction, where an acid and a base react to form water and a. Titration Labelled Diagram.
From www.science-revision.co.uk
Titrations Titration Labelled Diagram The end point of a titration is the point at. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. In each case, you start with 25. The figure below shows two different examples of a strong acid. — there are two basic types of. Titration Labelled Diagram.
From bramblechemistry.weebly.com
4C6 Titration Titration Labelled Diagram In each case, you start with 25. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. The figure below shows two different. Titration Labelled Diagram.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Labelled Diagram In each case, you start with 25. The figure below shows two different examples of a strong acid. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. . Titration Labelled Diagram.
From exoxjybnj.blob.core.windows.net
Acid Base Titration Lab Introduction at Nichole Brumback blog Titration Labelled Diagram titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The end point of a titration is the point at. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure. Titration Labelled Diagram.
From mungfali.com
Acid Base Titration Procedure Titration Labelled Diagram titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for. Titration Labelled Diagram.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Labelled Diagram The figure below shows two different examples of a strong acid. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The. Titration Labelled Diagram.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Labelled Diagram titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The end point of a titration is the point at. The figure below shows two different examples of a strong acid. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator. Titration Labelled Diagram.
From mavink.com
Titration Diagram Labeled Titration Labelled Diagram a titration curve is a graphical representation of the ph of a solution during a titration. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Labelled Diagram.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Labelled Diagram a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. — the shape of a titration curve, a plot of ph versus the amount of acid or base. Titration Labelled Diagram.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Labelled Diagram — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. In each case, you start with 25. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It is based on the neutralization. Titration Labelled Diagram.
From www.youtube.com
Titration tube How to draw well labelled Titration Tube Titration Titration Labelled Diagram The end point of a titration is the point at. — there are two basic types of acid base titrations, indicator and potentiometric. The figure below shows two different examples of a strong acid. In each case, you start with 25. It is based on the neutralization reaction, where an acid and a base react to form water and. Titration Labelled Diagram.
From mungfali.com
Acid Base Titration Calculation Titration Labelled Diagram The end point of a titration is the point at. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. a titration curve is a graphical representation of the ph of a solution during a titration. It is based on the neutralization reaction, where an acid. Titration Labelled Diagram.
From philschatz.com
AcidBase Titrations · Chemistry Titration Labelled Diagram titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — there are two basic types of acid base titrations, indicator. Titration Labelled Diagram.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration Labelled Diagram The end point of a titration is the point at. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a titration curve is a graphical representation of the ph of a solution during a titration. the titration curves shown in figure 14.20 illustrate. Titration Labelled Diagram.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Labelled Diagram — there are two basic types of acid base titrations, indicator and potentiometric. In each case, you start with 25. The figure below shows two different examples of a strong acid. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. titration involves the gradual. Titration Labelled Diagram.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Labelled Diagram — there are two basic types of acid base titrations, indicator and potentiometric. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. The figure below shows two different examples of a strong acid. titration involves the gradual addition of a reagent of known concentration,. Titration Labelled Diagram.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Labelled Diagram — there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. —. Titration Labelled Diagram.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Labelled Diagram In each case, you start with 25. — the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration. Titration Labelled Diagram.