Copper Ii Oxide Sulfuric Acid Balanced Equation . The copper takes the [o] and become cuo. The copper oxide and sulphuric acid balanced equation is given below: Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. What is the balanced equation for copper oxide and sulphuric acid? Why do copper oxide and sulphuric acid turn blue? You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The symbol equation for the reaction is: The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The balanced equation will appear. Double replacement reaction, proved by the formation of either a liquid or gas.
from www.advance-africa.com
The balanced equation will appear. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The copper takes the [o] and become cuo. What is the balanced equation for copper oxide and sulphuric acid? The copper oxide and sulphuric acid balanced equation is given below: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Double replacement reaction, proved by the formation of either a liquid or gas. The symbol equation for the reaction is:
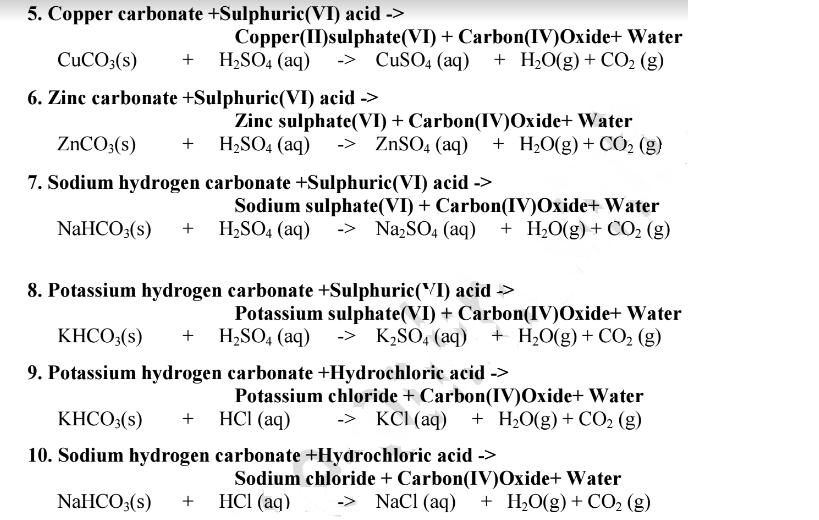
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests
Copper Ii Oxide Sulfuric Acid Balanced Equation Why do copper oxide and sulphuric acid turn blue? The copper oxide and sulphuric acid balanced equation is given below: The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The copper takes the [o] and become cuo. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The symbol equation for the reaction is: What is the balanced equation for copper oxide and sulphuric acid? You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Double replacement reaction, proved by the formation of either a liquid or gas. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Why do copper oxide and sulphuric acid turn blue?
From www.sciencephoto.com
Copper (II) oxide and sulphuric acid Stock Image C037/2520 Copper Ii Oxide Sulfuric Acid Balanced Equation What is the balanced equation for copper oxide and sulphuric acid? To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The copper oxide and sulphuric acid balanced equation is given below: The balanced. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.slideserve.com
PPT Chapter 6 OxidationReduction Reactions PowerPoint Presentation Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) Double replacement reaction, proved by the formation of either a liquid or gas. The copper takes the [o] and become cuo. The copper oxide and sulphuric acid balanced equation is given below: The symbol equation for the reaction is: Why do copper oxide and sulphuric acid turn. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations to correspond to each of the Copper Ii Oxide Sulfuric Acid Balanced Equation The copper oxide and sulphuric acid balanced equation is given below: The symbol equation for the reaction is: The balanced equation will appear. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The copper takes the [o] and become cuo. Why do copper oxide and sulphuric acid turn blue? To balance a chemical equation, enter an. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From kristophernpetton.blogspot.com
Copper Oxide and Sulfuric Acid Formula KristophernPetton Copper Ii Oxide Sulfuric Acid Balanced Equation Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Double replacement reaction, proved by the formation of either a liquid or gas. The copper oxide and sulphuric acid balanced equation is given below: Why do copper oxide and sulphuric acid turn blue? To balance a chemical equation, enter an equation of a chemical reaction and. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED When sulfuric acid and copper (II) oxide are allowed to react Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The symbol equation for the reaction is: The copper oxide and. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From kristophernpetton.blogspot.com
Copper Oxide and Sulfuric Acid Formula KristophernPetton Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation will appear. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The symbol equation for the reaction is: The copper oxide and sulphuric acid balanced equation is given below: Double replacement reaction, proved by the formation. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Convert the following word equations to balanced chemical Copper Ii Oxide Sulfuric Acid Balanced Equation You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The balanced equation will appear. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The symbol equation for the reaction is: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Solved and balanced chemical. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Ii Oxide Sulfuric Acid Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The symbol equation for the reaction is: The copper oxide and sulphuric acid balanced equation is given below: Why do copper oxide and sulphuric acid turn blue? Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The copper. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.sciencephoto.com
Copper (II) oxide reacts with sulfuric acid Stock Image C036/3130 Copper Ii Oxide Sulfuric Acid Balanced Equation The copper oxide and sulphuric acid balanced equation is given below: You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The copper takes the [o] and become cuo. The. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper Ii Oxide Sulfuric Acid Balanced Equation Why do copper oxide and sulphuric acid turn blue? Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The copper oxide and sulphuric acid. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Ii Oxide Sulfuric Acid Balanced Equation Why do copper oxide and sulphuric acid turn blue? You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. To balance a chemical equation,. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation will appear. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The copper takes the [o] and become cuo. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The symbol equation for the reaction is: Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient.. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Copper Ii Oxide Sulfuric Acid Balanced Equation The copper takes the [o] and become cuo. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Why do copper oxide and sulphuric acid turn blue? You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. What is the balanced equation for copper oxide and sulphuric acid? Solved and. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED 4. Write balanced chemical equations for each of the reactions Copper Ii Oxide Sulfuric Acid Balanced Equation Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The copper takes the [o] and become cuo. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. The balanced equation will appear. Why. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.slideshare.net
Acids And Bases Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) Double replacement reaction, proved by the formation of either a liquid or gas. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The copper takes the [o] and become cuo. The copper oxide and sulphuric acid balanced equation is given below: What. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equations for the reactions of sulfuric acid Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Why do copper oxide and sulphuric acid turn blue? The balanced equation will appear. What is the balanced equation for copper oxide and sulphuric acid? Double replacement reaction, proved by the formation of. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.youtube.com
Balancing Chemical Equations. Part 2 Sulfuric Acid and Sodium Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation will appear. What is the balanced equation for copper oxide and sulphuric acid? You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Double replacement reaction, proved by the formation of either a liquid or. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.meritnation.com
balance and write symbol of copper+sulphuric acid gives copper(2 Copper Ii Oxide Sulfuric Acid Balanced Equation The copper takes the [o] and become cuo. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. What is the balanced equation for copper oxide and sulphuric acid? The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) You can imagine concentrated sulfuric acid. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From daisy-yersblogchoi.blogspot.com
Word Equation for Copper Oxide and Sulfuric Acid Copper Ii Oxide Sulfuric Acid Balanced Equation The copper takes the [o] and become cuo. The copper oxide and sulphuric acid balanced equation is given below: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. What is the balanced equation for copper oxide and sulphuric acid? Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Q.G) Write the balanced chemical equations of the following Copper Ii Oxide Sulfuric Acid Balanced Equation Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The copper oxide and sulphuric acid. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations for each reaction and Copper Ii Oxide Sulfuric Acid Balanced Equation What is the balanced equation for copper oxide and sulphuric acid? To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The copper takes the [o] and become cuo. The balanced equation will appear. Double replacement reaction, proved by the formation of either a liquid or gas. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l). Copper Ii Oxide Sulfuric Acid Balanced Equation.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Ii Oxide Sulfuric Acid Balanced Equation The symbol equation for the reaction is: Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Double replacement reaction, proved by the formation of either a liquid or gas. The copper oxide and sulphuric acid balanced equation is given below: What is the balanced equation for copper oxide and sulphuric acid? To balance a chemical. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.youtube.com
neutralisation of sulphuric acid with copper oxide.mp4 YouTube Copper Ii Oxide Sulfuric Acid Balanced Equation What is the balanced equation for copper oxide and sulphuric acid? Why do copper oxide and sulphuric acid turn blue? The balanced equation will appear. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. To balance. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From hxeoymgas.blob.core.windows.net
Copper Oxide + Sulphuric Acid Balanced Equation at John Crockett blog Copper Ii Oxide Sulfuric Acid Balanced Equation The copper oxide and sulphuric acid balanced equation is given below: Double replacement reaction, proved by the formation of either a liquid or gas. Why do copper oxide and sulphuric acid turn blue? Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The copper takes the [o] and become cuo. Solved and balanced chemical equation. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Word Equation Balanced Chemical Equation When solid copper Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The copper takes the [o] and become cuo. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Why do copper oxide and sulphuric acid turn blue? Double replacement reaction, proved by the formation of either a liquid or gas. To balance a chemical. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Copper Ii Oxide Sulfuric Acid Balanced Equation Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The balanced equation will appear. To balance a chemical equation, enter an equation of a. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Ii Oxide Sulfuric Acid Balanced Equation What is the balanced equation for copper oxide and sulphuric acid? The symbol equation for the reaction is: Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Double replacement reaction, proved by the formation of either. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052 Copper Ii Oxide Sulfuric Acid Balanced Equation You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. The balanced equation will appear. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) What is the balanced equation for copper oxide and sulphuric. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED I am required to find the molecular equation, ionic equation Copper Ii Oxide Sulfuric Acid Balanced Equation The balanced equation will appear. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) Double replacement reaction, proved by the formation of either a liquid or gas. The symbol equation for the reaction is: What is the balanced equation for copper oxide and sulphuric acid? Why do copper oxide and sulphuric acid turn blue? To balance. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From hxeoymgas.blob.core.windows.net
Copper Oxide + Sulphuric Acid Balanced Equation at John Crockett blog Copper Ii Oxide Sulfuric Acid Balanced Equation Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. What is the balanced equation for copper oxide and sulphuric acid? The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l). Copper Ii Oxide Sulfuric Acid Balanced Equation.
From aptaste.weebly.com
Sulfuric acid formula balance chemical equations calculator aptaste Copper Ii Oxide Sulfuric Acid Balanced Equation The copper oxide and sulphuric acid balanced equation is given below: The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) Why do copper oxide and sulphuric acid turn blue? The balanced equation will appear. What is the balanced equation for copper oxide and sulphuric acid? You can imagine concentrated sulfuric acid as an oxidising agent which. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.chegg.com
Solved When sulfuric acid and copper (II) oxide are allowed Copper Ii Oxide Sulfuric Acid Balanced Equation Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) The balanced equation will appear. Why do copper oxide and sulphuric acid turn blue? Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products.. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper Ii Oxide Sulfuric Acid Balanced Equation The symbol equation for the reaction is: The copper takes the [o] and become cuo. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Why do copper oxide and sulphuric acid turn blue? Double replacement reaction, proved by the formation of either a liquid or gas. The balanced equation will appear. The balanced equation for this. Copper Ii Oxide Sulfuric Acid Balanced Equation.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Copper Ii Oxide Sulfuric Acid Balanced Equation Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. The balanced equation will appear. Cuo(s) + h2so4(aq) cuso4(aq) + h2o(l) otherwise, a simple word equation will be sufficient. Why do copper oxide and sulphuric acid turn blue? The balanced equation for this reaction is cuo(s) + h2so4(aq) cuso4(aq) + h2o(l). Copper Ii Oxide Sulfuric Acid Balanced Equation.
From tanianewsbridges.blogspot.com
Copper Oxide Sulfuric Acid Copper Ii Oxide Sulfuric Acid Balanced Equation The symbol equation for the reaction is: Double replacement reaction, proved by the formation of either a liquid or gas. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The copper takes the [o] and become cuo. You can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Cuo(s) +. Copper Ii Oxide Sulfuric Acid Balanced Equation.