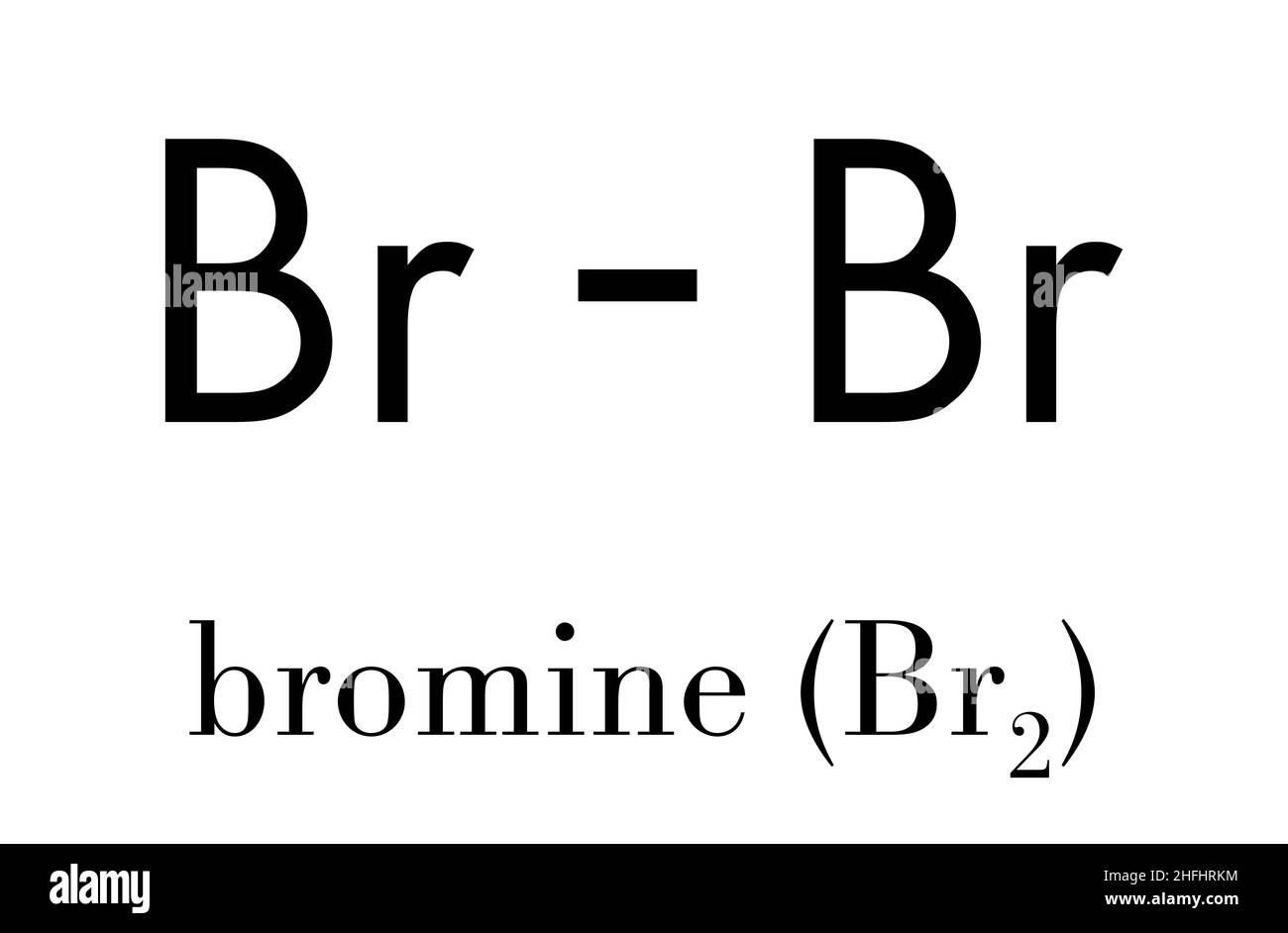Draw A Lewis Dot Structure For Bromine . Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculating the total number of. Draw a lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence. How to draw lewis structure for bromine. It is very easy to br 2 lewis structure. Drawing lewis structure for bromine. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There are a few steps that need to be followed to attain the stable and correct lewis structure which. To draw a stable and correct lewis structure, there are certain steps that need to be followed:
from ar.inspiredpencil.com
Drawing lewis structure for bromine. Draw a lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence. Calculating the total number of. How to draw lewis structure for bromine. It is very easy to br 2 lewis structure. There are a few steps that need to be followed to attain the stable and correct lewis structure which. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2.
Br2 Lewis Structure
Draw A Lewis Dot Structure For Bromine To draw a stable and correct lewis structure, there are certain steps that need to be followed: There are a few steps that need to be followed to attain the stable and correct lewis structure which. Drawing lewis structure for bromine. It is very easy to br 2 lewis structure. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Calculating the total number of. Draw a lewis electron dot diagram for an atom or a monatomic ion. How to draw lewis structure for bromine. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. In almost all cases, chemical bonds are formed by interactions of valence.
From lambdageeks.com
Barium Lewis Dot Structure Drawing, Several Compounds and Detailed Draw A Lewis Dot Structure For Bromine There are a few steps that need to be followed to attain the stable and correct lewis structure which. It is very easy to br 2 lewis structure. Calculating the total number of. In almost all cases, chemical bonds are formed by interactions of valence. There is a single bond with bromine atoms and three lone pairs on each bromine. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
Lewis Dot Structure for BoronHow do you draw the Lewis Dot structure Draw A Lewis Dot Structure For Bromine Draw a lewis electron dot diagram for an atom or a monatomic ion. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculating the total number of. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. How to draw lewis structure for bromine.. Draw A Lewis Dot Structure For Bromine.
From quizlet.com
Draw the Lewis dot structure for boron trichloride, BCl_3. Quizlet Draw A Lewis Dot Structure For Bromine Draw a lewis electron dot diagram for an atom or a monatomic ion. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Calculating the total number of. It is very easy. Draw A Lewis Dot Structure For Bromine.
From loeyjrpcp.blob.core.windows.net
Bromine Dot Structure at Marie Priddy blog Draw A Lewis Dot Structure For Bromine Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There are a few steps that need to be followed to attain the stable and correct lewis structure which. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculating the total number of. To. Draw A Lewis Dot Structure For Bromine.
From dxofadauo.blob.core.windows.net
How To Draw A Dot Diagram at Juanita Stark blog Draw A Lewis Dot Structure For Bromine Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. In almost all cases, chemical bonds are formed by interactions of valence. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. How to draw lewis structure for bromine. Calculate formal charges and use the electroneutrality principle to. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
How to Draw the Lewis Dot Structure for CaBr2 Calcium bromide YouTube Draw A Lewis Dot Structure For Bromine It is very easy to br 2 lewis structure. There are a few steps that need to be followed to attain the stable and correct lewis structure which. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br. Draw A Lewis Dot Structure For Bromine.
From animalia-life.club
N2o4 Lewis Dot Structure Draw A Lewis Dot Structure For Bromine Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Calculating the total number of. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. To draw a stable. Draw A Lewis Dot Structure For Bromine.
From mavink.com
Lewis Structure For Bromine Draw A Lewis Dot Structure For Bromine It is very easy to br 2 lewis structure. To draw a stable and correct lewis structure, there are certain steps that need to be followed: There are a few steps that need to be followed to attain the stable and correct lewis structure which. How to draw lewis structure for bromine. Bromine lewis dot structure represents that bromine is. Draw A Lewis Dot Structure For Bromine.
From brainly.com
Draw the Lewis structures for Calcium bromide, CaBr2 Draw A Lewis Dot Structure For Bromine Drawing lewis structure for bromine. How to draw lewis structure for bromine. Draw a lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There is a single. Draw A Lewis Dot Structure For Bromine.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Draw A Lewis Dot Structure For Bromine It is very easy to br 2 lewis structure. Draw a lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. Drawing lewis structure for bromine. To draw. Draw A Lewis Dot Structure For Bromine.
From mungfali.com
Draw Lewis Structure Draw A Lewis Dot Structure For Bromine Drawing lewis structure for bromine. Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. In almost all cases, chemical bonds are formed by interactions of valence. How to draw lewis structure for bromine. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There. Draw A Lewis Dot Structure For Bromine.
From jkd-fotografie.blogspot.com
Chlorine Pentafluoride Lewis Dot Diagram First draw the lewis dot Draw A Lewis Dot Structure For Bromine Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. Calculating the total number of. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. How to draw lewis structure for bromine. In almost all cases, chemical bonds are formed by interactions of valence. It. Draw A Lewis Dot Structure For Bromine.
From www.coursehero.com
[Solved] Construct a Lewis structure for potassium and bromine. Predict Draw A Lewis Dot Structure For Bromine Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. There are a few steps that need to be followed to attain the stable and correct lewis structure which. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Drawing lewis structure for bromine. How to draw. Draw A Lewis Dot Structure For Bromine.
From www.numerade.com
SOLVEDGermanium, antimony, selenium, and bromine each bond to a Draw A Lewis Dot Structure For Bromine It is very easy to br 2 lewis structure. To draw a stable and correct lewis structure, there are certain steps that need to be followed: In almost all cases, chemical bonds are formed by interactions of valence. Draw a lewis electron dot diagram for an atom or a monatomic ion. Drawing lewis structure for bromine. Bromine lewis dot structure. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
How to Draw the Lewis Dot Structure for LiBr Lithium bromide YouTube Draw A Lewis Dot Structure For Bromine Draw a lewis electron dot diagram for an atom or a monatomic ion. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. In almost all cases, chemical bonds are formed by interactions of valence.. Draw A Lewis Dot Structure For Bromine.
From mavink.com
Mg Lewis Structure Draw A Lewis Dot Structure For Bromine There are a few steps that need to be followed to attain the stable and correct lewis structure which. Draw a lewis electron dot diagram for an atom or a monatomic ion. It is very easy to br 2 lewis structure. Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. Calculate formal charges. Draw A Lewis Dot Structure For Bromine.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Draw A Lewis Dot Structure For Bromine There are a few steps that need to be followed to attain the stable and correct lewis structure which. Calculating the total number of. In almost all cases, chemical bonds are formed by interactions of valence. How to draw lewis structure for bromine. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculate. Draw A Lewis Dot Structure For Bromine.
From www.chegg.com
Solved 3 points 28) What is the correct Electron Dot Diagram Draw A Lewis Dot Structure For Bromine To draw a stable and correct lewis structure, there are certain steps that need to be followed: How to draw lewis structure for bromine. It is very easy to br 2 lewis structure. Drawing lewis structure for bromine. In almost all cases, chemical bonds are formed by interactions of valence. Bromine lewis dot structure represents that bromine is a diatomic. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
Lewis Structure of AlBr3, Aluminum Bromide YouTube Draw A Lewis Dot Structure For Bromine Draw a lewis electron dot diagram for an atom or a monatomic ion. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Calculating the total number of. There are a few steps that need to be followed to attain the stable and correct lewis structure which. In almost all cases, chemical bonds. Draw A Lewis Dot Structure For Bromine.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Draw A Lewis Dot Structure For Bromine Draw a lewis electron dot diagram for an atom or a monatomic ion. Calculating the total number of. Drawing lewis structure for bromine. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or. Draw A Lewis Dot Structure For Bromine.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Draw A Lewis Dot Structure For Bromine It is very easy to br 2 lewis structure. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Drawing lewis structure for bromine. In almost all cases, chemical bonds are formed by interactions of valence. Draw a lewis electron dot diagram for an atom or a monatomic ion. Bromine lewis dot structure represents. Draw A Lewis Dot Structure For Bromine.
From hanenhuusholli.blogspot.com
Lewis Dot Diagram For Bromine Hanenhuusholli Draw A Lewis Dot Structure For Bromine Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. How to draw lewis structure for bromine. To draw a stable and correct lewis structure, there are certain steps that need to be followed: There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculating the total number. Draw A Lewis Dot Structure For Bromine.
From exoincqad.blob.core.windows.net
Lewis Dot Structure Of Carbon And Bromine at Linda Stone blog Draw A Lewis Dot Structure For Bromine Drawing lewis structure for bromine. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculating the total number of. Draw a lewis electron dot diagram for an atom or a monatomic ion. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. To draw. Draw A Lewis Dot Structure For Bromine.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Draw A Lewis Dot Structure For Bromine Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There are a few steps that need to be followed to attain the stable and correct lewis structure which. How to draw lewis structure for bromine. There is a single bond with bromine atoms and three lone pairs on each bromine. Draw A Lewis Dot Structure For Bromine.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Draw A Lewis Dot Structure For Bromine There are a few steps that need to be followed to attain the stable and correct lewis structure which. Draw a lewis electron dot diagram for an atom or a monatomic ion. It is very easy to br 2 lewis structure. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Drawing lewis. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
Draw the Lewis Structure of CaBr2 (Calcium bromide) YouTube Draw A Lewis Dot Structure For Bromine Calculating the total number of. Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. It is very easy to br 2 lewis structure. To draw a stable and correct lewis structure, there are certain steps that need to be followed: Draw a lewis electron dot diagram for an atom or a monatomic ion.. Draw A Lewis Dot Structure For Bromine.
From ar.inspiredpencil.com
Br2 Lewis Structure Draw A Lewis Dot Structure For Bromine In almost all cases, chemical bonds are formed by interactions of valence. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Drawing lewis structure for bromine. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. To draw a stable and correct lewis structure,. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br (Bromide ion) YouTube Draw A Lewis Dot Structure For Bromine Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. In almost all cases, chemical bonds are formed by interactions of valence. Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. Drawing lewis structure for bromine. It is very easy to br 2 lewis. Draw A Lewis Dot Structure For Bromine.
From loeyjrpcp.blob.core.windows.net
Bromine Dot Structure at Marie Priddy blog Draw A Lewis Dot Structure For Bromine To draw a stable and correct lewis structure, there are certain steps that need to be followed: There is a single bond with bromine atoms and three lone pairs on each bromine atoms. There are a few steps that need to be followed to attain the stable and correct lewis structure which. In almost all cases, chemical bonds are formed. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
Draw the Lewis Structure of MgBr2 (magnesium bromide) YouTube Draw A Lewis Dot Structure For Bromine Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. How to draw lewis structure for bromine. In almost all cases, chemical bonds are formed by interactions of valence. Calculating the total number of. Draw. Draw A Lewis Dot Structure For Bromine.
From www.numerade.com
SOLVED Draw the Lewis structure for a bromide monolodide (Brl) molecule. Draw A Lewis Dot Structure For Bromine Drawing lewis structure for bromine. There are a few steps that need to be followed to attain the stable and correct lewis structure which. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Draw a lewis electron dot diagram for an atom or a monatomic ion. Calculate formal charges and use the electroneutrality. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
Lewis Structure of Iron (II) Bromide, FeBr2 YouTube Draw A Lewis Dot Structure For Bromine Calculating the total number of. In almost all cases, chemical bonds are formed by interactions of valence. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. To draw a stable and. Draw A Lewis Dot Structure For Bromine.
From www.youtube.com
Lewis Diagrams Made Easy How to Draw Lewis Dot Structures YouTube Draw A Lewis Dot Structure For Bromine Drawing lewis structure for bromine. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. Bromine lewis dot structure represents that bromine is a diatomic molecule with the formula br 2. How. Draw A Lewis Dot Structure For Bromine.
From quizlet.com
Draw a Lewis dot structure and a structural formula for eac Quizlet Draw A Lewis Dot Structure For Bromine Calculating the total number of. There is a single bond with bromine atoms and three lone pairs on each bromine atoms. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. How to draw lewis structure for bromine. Draw a lewis electron dot diagram for an atom or a monatomic ion.. Draw A Lewis Dot Structure For Bromine.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Bromine General Wiring Diagram Draw A Lewis Dot Structure For Bromine Calculating the total number of. In almost all cases, chemical bonds are formed by interactions of valence. Draw a lewis electron dot diagram for an atom or a monatomic ion. Calculate formal charges and use the electroneutrality principle to determine which lewis dot structure is the best, or which. It is very easy to br 2 lewis structure. How to. Draw A Lewis Dot Structure For Bromine.