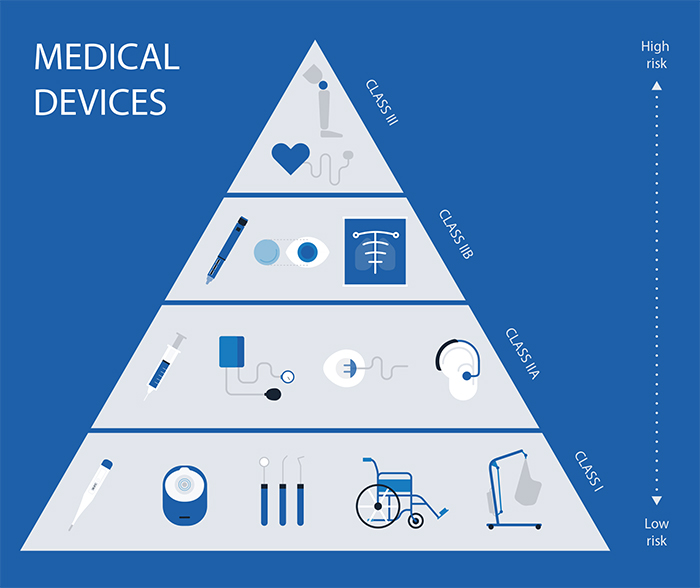
Medical Device Classes Explained . All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. Health canada’s classification system for medical devices explained. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Class iii are the least common class for medical. The fda has classified over 1,700 distinct types of medical devices. Health canada classifies all medical devices. Here are the 3 classes of devices and how the fda validates that they are safe and effective. From class i to iv: In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class i medical devices are the. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. Differences between fda medical device classes. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. The devices are organized in the code of federal regulations.
from laegemiddelstyrelsen.dk
Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. From class i to iv: In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. Health canada classifies all medical devices. Class i medical devices are the. The devices are organized in the code of federal regulations. Health canada’s classification system for medical devices explained. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. The fda has classified over 1,700 distinct types of medical devices. All medical device classes in the eu require working with a notified body, except for those which are class i and can be.
Medical devices
Medical Device Classes Explained From class i to iv: Differences between fda medical device classes. Here are the 3 classes of devices and how the fda validates that they are safe and effective. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Class iii are the least common class for medical. Class i medical devices are the. The devices are organized in the code of federal regulations. The fda has classified over 1,700 distinct types of medical devices. From class i to iv: In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. Health canada’s classification system for medical devices explained. Health canada classifies all medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance.
From www.researchgate.net
Medical Device Classification System Download Table Medical Device Classes Explained All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Differences between fda medical device classes. Class iii are the least common class for medical. Health canada classifies all medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk. Medical Device Classes Explained.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Classes Explained The fda has classified over 1,700 distinct types of medical devices. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. Class ii medical devices,. Medical Device Classes Explained.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Classes Explained Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. The fda has classified over 1,700 distinct types of medical devices. Health canada’s classification system for medical devices explained. Here are the 3 classes of devices and how the fda validates that they are. Medical Device Classes Explained.
From mungfali.com
Classification Of Medical Devices Medical Device Classes Explained From class i to iv: In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. The devices are organized in the code of federal regulations. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards. Medical Device Classes Explained.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Classes Explained From class i to iv: In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. The fda has classified over 1,700 distinct types of medical devices. Health canada’s classification system for medical devices explained. Health canada classifies all medical devices. The devices are organized in the code of. Medical Device Classes Explained.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Medical Device Classes Explained The devices are organized in the code of federal regulations. Class i medical devices are the. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance. Medical Device Classes Explained.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Classes Explained In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class i medical devices are the. The fda has classified over 1,700 distinct types of medical devices. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive. Medical Device Classes Explained.
From www.presentationeze.com
Validation Training.PresentationEZE Medical Device Classes Explained Health canada classifies all medical devices. Differences between fda medical device classes. Here are the 3 classes of devices and how the fda validates that they are safe and effective. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. In the u.s., the fda defines a. Medical Device Classes Explained.
From www.volersystems.com
Are You Making a Medical Device? Voler Systems Medical Device Classes Explained Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. Class i medical devices are the. Differences between fda medical device classes. Health canada classifies all medical devices. The devices are organized in the code of federal regulations. In canada, medical devices are grouped. Medical Device Classes Explained.
From advisera.com
FDA medical device classes Examples & ISO 13485 usage Medical Device Classes Explained All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. Here are the 3 classes of devices and how the fda. Medical Device Classes Explained.
From mdrc-consulting.com
General medical devices, medical equipment MDRC Medical Device Classes Explained Differences between fda medical device classes. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. From class i to iv: Class iii are the least common class for medical. The devices are organized in the code of federal regulations. Health canada’s classification system for medical devices explained.. Medical Device Classes Explained.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classes Explained The devices are organized in the code of federal regulations. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. Differences between fda medical device classes. Class iii are the least common class for medical. In canada, medical devices are grouped into 4 classes. Medical Device Classes Explained.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classes Explained Class i medical devices are the. Class iii are the least common class for medical. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. All medical device classes in the eu require working with a notified body, except for those which are class i and can be.. Medical Device Classes Explained.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Classes Explained From class i to iv: In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Health canada’s classification system for medical devices explained. Differences between fda medical device classes. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm. Medical Device Classes Explained.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Classes Explained Health canada classifies all medical devices. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. In the u.s., the fda defines. Medical Device Classes Explained.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classes Explained The devices are organized in the code of federal regulations. From class i to iv: Health canada’s classification system for medical devices explained. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. The fda has classified over 1,700 distinct types of medical devices. Here are the. Medical Device Classes Explained.
From www.slideserve.com
PPT Therapeutic Goods Administration TGA PowerPoint Presentation Medical Device Classes Explained Health canada classifies all medical devices. Class i medical devices are the. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. Health canada’s classification system for medical devices explained. The devices are organized in the code of federal regulations. All medical device classes in the eu require. Medical Device Classes Explained.
From sunstonepilot.com
Introduction to Medical Device Development Sunstone Pilot, Inc. Medical Device Classes Explained In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. Class i medical devices are the. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Health canada classifies all medical devices. Class iii are. Medical Device Classes Explained.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Medical Device Classes Explained From class i to iv: All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Class i medical devices are the. Differences between fda medical device classes. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health. Medical Device Classes Explained.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Classes Explained The devices are organized in the code of federal regulations. The fda has classified over 1,700 distinct types of medical devices. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class iii are the least common class for medical. Differences between fda medical device classes. From class. Medical Device Classes Explained.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Classes Explained Health canada classifies all medical devices. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. The devices are organized in the code of federal regulations. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and. Medical Device Classes Explained.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classes Explained In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Differences between fda medical device classes. Health canada classifies all medical devices. Health canada’s classification system for medical devices explained. In the u.s., the fda defines a class iii medical device as a device that presents severe potential. Medical Device Classes Explained.
From www.youtube.com
Medical Device Classes Explained A Beginner's Guide YouTube Medical Device Classes Explained In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class i medical devices are the. The fda has classified over 1,700 distinct types of medical devices. All medical device classes in the eu require working with a notified body, except for those which are class i and. Medical Device Classes Explained.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Classes Explained In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. Here are the 3 classes of devices and how the fda validates. Medical Device Classes Explained.
From japanhpn.org
Japan Health Policy NOW 6.4 Medical Devices Medical Device Classes Explained Health canada’s classification system for medical devices explained. From class i to iv: In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Class iii. Medical Device Classes Explained.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Classes Explained Class iii are the least common class for medical. Here are the 3 classes of devices and how the fda validates that they are safe and effective. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. Class i medical devices are the. Health canada’s classification system. Medical Device Classes Explained.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? Medical Device Classes Explained Health canada classifies all medical devices. Class iii are the least common class for medical. Here are the 3 classes of devices and how the fda validates that they are safe and effective. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. In canada, medical devices are. Medical Device Classes Explained.
From www.youtube.com
Medical Device Classes YouTube Medical Device Classes Explained In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. Class iii are the least common class for medical. Health canada’s classification system for medical. Medical Device Classes Explained.
From www.medisurge.com
Understanding Medical Device Classes MEDISURGE Medical Device Classes Explained The devices are organized in the code of federal regulations. All medical device classes in the eu require working with a notified body, except for those which are class i and can be. The fda has classified over 1,700 distinct types of medical devices. From class i to iv: In canada, medical devices are grouped into 4 classes based on. Medical Device Classes Explained.
From medium.com
The 3 FDA medical device classes [differences and examples explained Medical Device Classes Explained Health canada’s classification system for medical devices explained. Class i medical devices are the. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards. Medical Device Classes Explained.
From mavink.com
Fda Medical Device Classification Chart Medical Device Classes Explained Health canada’s classification system for medical devices explained. In canada, medical devices are grouped into 4 classes based on the expected level of risk to a person's health and safety. In the u.s., the fda defines a class iii medical device as a device that presents severe potential harm to the individual. From class i to iv: Differences between fda. Medical Device Classes Explained.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Classes Explained All medical device classes in the eu require working with a notified body, except for those which are class i and can be. From class i to iv: Differences between fda medical device classes. Class iii are the least common class for medical. The devices are organized in the code of federal regulations. Here are the 3 classes of devices. Medical Device Classes Explained.
From www.pacificbridgemedical.com
Device Classification in India Infographic Medical Device Classes Explained The devices are organized in the code of federal regulations. Here are the 3 classes of devices and how the fda validates that they are safe and effective. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. Class ii medical devices, also known as class ii. Medical Device Classes Explained.
From operonstrategist.com
Classifying a Class III Medical Device Process) Operon Medical Device Classes Explained Class ii medical devices, also known as class ii devices, must comply with general controls and special controls, which may include performance standards & postmarket surveillance. Health canada classifies all medical devices. The devices are organized in the code of federal regulations. Health canada’s classification system for medical devices explained. Class iii are the least common class for medical. In. Medical Device Classes Explained.
From www.qualio.com
Medical device classification guide Medical Device Classes Explained Health canada classifies all medical devices. The fda has classified over 1,700 distinct types of medical devices. Differences between fda medical device classes. All medical devices to be sold in the european union must first obtain a ce marking, a process which requires extensive technical documentation. In canada, medical devices are grouped into 4 classes based on the expected level. Medical Device Classes Explained.