What Are The Three Categories Of Atomic Solids . Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids based on the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. They tend to be very hard and have high melting points. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids.
from stock.adobe.com
Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids based on the solid pack. They tend to be very hard and have high melting points.
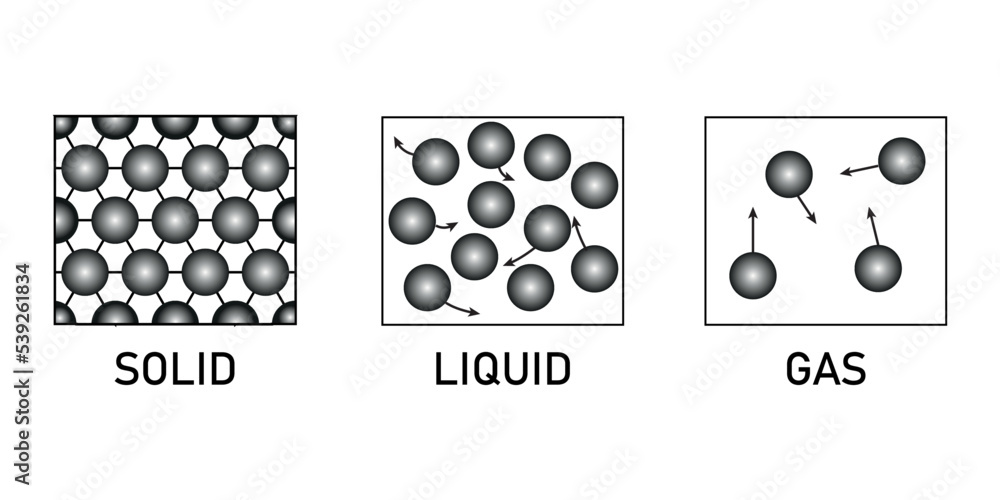
states of matter solids liquids and gases. Matter appears in three
What Are The Three Categories Of Atomic Solids Solids can be divided into three categories on the basis of how the particles that form the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Categories of solids based on the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Solids can be divided into three categories on the basis of how the particles that form the solid pack.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Categories of solids based on the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into three categories on. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5173446 What Are The Three Categories Of Atomic Solids Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. They tend to be very hard and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Solids can be. What Are The Three Categories Of Atomic Solids.
From www.numerade.com
SOLVEDWhatare the three Categories of atomic solids? Check all What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. They tend to be very hard and have high melting points. The three categories. What Are The Three Categories Of Atomic Solids.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Categories of solids. What Are The Three Categories Of Atomic Solids.
From www.askiitians.com
General Characteristics of Solid State Study Material for IIT JEE What Are The Three Categories Of Atomic Solids The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Categories of solids based on the solid pack. They tend to be very hard and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which. What Are The Three Categories Of Atomic Solids.
From www.nuclear-power.com
Atomic and Chemical Bond What Are The Three Categories Of Atomic Solids Categories of solids based on the solid pack. Solids can be divided into three categories on the basis of how the particles that form the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in. What Are The Three Categories Of Atomic Solids.
From www.sliderbase.com
Network Atomic Solids What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Solids can be divided into three categories on the basis of how the particles that form the solid pack. They tend to be very hard and have high melting points. The three categories. What Are The Three Categories Of Atomic Solids.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. The three categories of atomic solids are network (covalent) solids, metallic solids, and van. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID9479725 What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. They tend to be very hard and have high melting points. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Diamond is a network solid. What Are The Three Categories Of Atomic Solids.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids based on the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Ionic solids, such as sodium. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID608748 What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. They tend to be very hard and have high melting points. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids. What Are The Three Categories Of Atomic Solids.
From www.sliderbase.com
Network Atomic Solids What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Diamond is a network solid. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Structures and Types of Solids PowerPoint Presentation, free What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Solids can be divided into three categories on the basis of how the particles that form the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van. What Are The Three Categories Of Atomic Solids.
From wisc.pb.unizin.org
M11Q3 Types of Solids Chem 103/104 Resource Book What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Categories of solids based on the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Ionic solids, such as sodium. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Categories of solids based on the solid pack. They tend to be very hard and have. What Are The Three Categories Of Atomic Solids.
From www.numerade.com
⏩SOLVEDWhat are the three categories of atomic solids? Numerade What Are The Three Categories Of Atomic Solids Categories of solids based on the solid pack. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Diamond is a network solid and. What Are The Three Categories Of Atomic Solids.
From www.youtube.com
Ionic Solids, Molecular Solids, Metallic Solids, Network Covalent What Are The Three Categories Of Atomic Solids Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids based on the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. They tend to be very hard. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Structures and Types of Solids PowerPoint Presentation, free What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into three categories on the basis of how the. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID9479725 What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Solids can be divided into three categories on the basis of how the particles that form the solid pack. They tend to be very hard and have high melting points. Categories of solids. What Are The Three Categories Of Atomic Solids.
From www.sliderbase.com
Network Atomic Solids What Are The Three Categories Of Atomic Solids The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Categories of solids based on the solid pack. They tend to be very hard and have high melting points. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Ionic solids, such as sodium. What Are The Three Categories Of Atomic Solids.
From saylordotorg.github.io
The Arrangement of Atoms in Crystalline Solids What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into. What Are The Three Categories Of Atomic Solids.
From chem.libretexts.org
12.2 The Arrangement of Atoms in Crystalline Solids Chemistry LibreTexts What Are The Three Categories Of Atomic Solids Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Categories of solids based on the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. The three categories of atomic solids. What Are The Three Categories Of Atomic Solids.
From www.app.formative.com
Types of Solids Caroline Duesing Library Formative What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids based on the solid pack. The three categories of atomic solids. What Are The Three Categories Of Atomic Solids.
From shaunmwilliams.com
Chapter 10 Presentation What Are The Three Categories Of Atomic Solids Solids can be divided into three categories on the basis of how the particles that form the solid pack. They tend to be very hard and have high melting points. Categories of solids based on the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Structures and Types of Solids PowerPoint Presentation, free What Are The Three Categories Of Atomic Solids Categories of solids based on the solid pack. Solids can be divided into three categories on the basis of how the particles that form the solid pack. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. They tend to be very hard and have high melting points. Ionic solids, such as sodium. What Are The Three Categories Of Atomic Solids.
From schoolbag.info
FIGURE 12.1 Classifications of solids according to predominant bonding What Are The Three Categories Of Atomic Solids The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. They tend to be very hard and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Diamond is a network solid. What Are The Three Categories Of Atomic Solids.
From stock.adobe.com
states of matter solids liquids and gases. Matter appears in three What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Categories of solids based on the solid pack. Ionic solids, such as sodium. What Are The Three Categories Of Atomic Solids.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with What Are The Three Categories Of Atomic Solids Categories of solids based on the solid pack. They tend to be very hard and have high melting points. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Ionic solids, such as sodium. What Are The Three Categories Of Atomic Solids.
From www.slideserve.com
PPT Crystalline Solids PowerPoint Presentation, free download ID What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. They tend to be very hard and have high melting points. Diamond is a network solid. What Are The Three Categories Of Atomic Solids.
From www.numerade.com
What are the three categories of atomic solids? Numerade What Are The Three Categories Of Atomic Solids The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into three categories on the basis of how the particles that form the solid pack. They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to. What Are The Three Categories Of Atomic Solids.
From www.sliderbase.com
Network Atomic Solids What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. Solids can be divided into. What Are The Three Categories Of Atomic Solids.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science What Are The Three Categories Of Atomic Solids Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Categories of solids based on. What Are The Three Categories Of Atomic Solids.
From www.vecteezy.com
Different models of atom vector illustration 21669330 Vector Art at What Are The Three Categories Of Atomic Solids Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite. They tend to be very hard and have high melting points. The three categories of atomic solids are network (covalent) solids, metallic solids, and van der waals solids. Solids can be divided into. What Are The Three Categories Of Atomic Solids.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Categories of solids based on the solid pack. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic. What Are The Three Categories Of Atomic Solids.
From www.chegg.com
Solved What are the three categories of atomic solids? Check What Are The Three Categories Of Atomic Solids They tend to be very hard and have high melting points. Categories of solids based on the solid pack. Solids can be divided into three categories on the basis of how the particles that form the solid pack. Diamond is a network solid and consists of carbon atoms covalently bonded to one another in a repeating three. Ionic solids, such. What Are The Three Categories Of Atomic Solids.