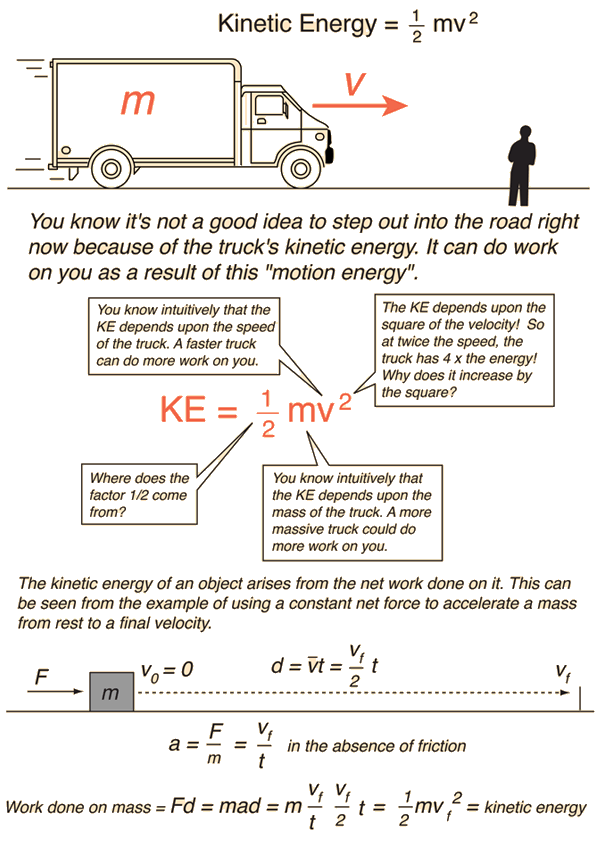
Kinetic Energy Situations . If an object is not moving, it. kinetic energy, form of energy that an object or a particle has by reason of its motion. This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. Kinetic energy is the energy that any object with mass has simply because it is moving. kinetic energy depends on the velocity of the object squared. If work, which transfers energy,. energy is an important concept in physics that is used to understand large and small processes.
from hyperphysics.phy-astr.gsu.edu
This means that when the velocity of an object doubles, its kinetic energy quadruples. kinetic energy depends on the velocity of the object squared. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. If an object is not moving, it. energy is an important concept in physics that is used to understand large and small processes. Kinetic energy is the energy that any object with mass has simply because it is moving. If work, which transfers energy,. kinetic energy, form of energy that an object or a particle has by reason of its motion.
Energy
Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. This means that when the velocity of an object doubles, its kinetic energy quadruples. energy is an important concept in physics that is used to understand large and small processes. If an object is not moving, it. kinetic energy depends on the velocity of the object squared. If work, which transfers energy,. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. kinetic energy, form of energy that an object or a particle has by reason of its motion. Kinetic energy is the energy that any object with mass has simply because it is moving. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,.
From kidsdiscover.com
Infographic Potential vs. Energy Kids Discover Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. kinetic energy, form of energy that an object or a particle has by reason of its motion. Kinetic energy is the energy that any object with mass has simply because it is moving. If work, which transfers energy,. kinetic energy depends on the velocity. Kinetic Energy Situations.
From oyehero.com
& Potential Difference energy in Hindi गतिज ऊर्जा और स्थितिज Kinetic Energy Situations kinetic energy depends on the velocity of the object squared. If an object is not moving, it. If work, which transfers energy,. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. This means that when the velocity of an object doubles, its kinetic energy quadruples. Kinetic energy is the energy that any object. Kinetic Energy Situations.
From www.englishworksheet.my.id
Potential Vs Energy Worksheet Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. kinetic energy, form of energy that an object or a particle has by reason of its motion. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21. Kinetic Energy Situations.
From www.bigstockphoto.com
Theory Matter Image & Photo (Free Trial) Bigstock Kinetic Energy Situations If an object is not moving, it. If work, which transfers energy,. Kinetic energy is the energy that any object with mass has simply because it is moving. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. energy is. Kinetic Energy Situations.
From steamism.com
The 2 types and 9 forms of Energy and Potential Kinetic Energy Situations energy is an important concept in physics that is used to understand large and small processes. If work, which transfers energy,. This means that when the velocity of an object doubles, its kinetic energy quadruples. If an object is not moving, it. kinetic energy depends on the velocity of the object squared. Kinetic energy is the energy that. Kinetic Energy Situations.
From cstephenmurray.com
Mr Murray's site Work and Energy Notes Kinetic Energy Situations kinetic energy, form of energy that an object or a particle has by reason of its motion. kinetic energy depends on the velocity of the object squared. This means that when the velocity of an object doubles, its kinetic energy quadruples. Kinetic energy is the energy that any object with mass has simply because it is moving. . Kinetic Energy Situations.
From sciencenotes.org
What Is Energy? Energy Examples Kinetic Energy Situations The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. This means that when the velocity of an object doubles, its kinetic energy quadruples. energy is an important concept in physics that is used to understand large and small processes.. Kinetic Energy Situations.
From www.bartleby.com
Energy and WorkEnergy Theorem bartleby Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. Kinetic energy is the energy that any object with mass has simply because it is moving. . Kinetic Energy Situations.
From kids.britannica.com
energy Kids Britannica Kids Homework Help Kinetic Energy Situations energy is an important concept in physics that is used to understand large and small processes. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and. Kinetic Energy Situations.
From armfield.co.uk
EF2.4 Dynamics Potential and Energy Armfield Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. Kinetic energy is the energy that any object with mass has simply because it is moving. . Kinetic Energy Situations.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 11 Work and Energy Part 1 Kinetic Energy Situations energy is an important concept in physics that is used to understand large and small processes. If work, which transfers energy,. Kinetic energy is the energy that any object with mass has simply because it is moving. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. This means that when the velocity of. Kinetic Energy Situations.
From www.pinterest.co.uk
Potential and Energy Worksheet Best Of "potential Vs Kinetic Energy Situations kinetic energy depends on the velocity of the object squared. kinetic energy, form of energy that an object or a particle has by reason of its motion. If an object is not moving, it. Kinetic energy is the energy that any object with mass has simply because it is moving. The average translational kinetic energy of a molecule. Kinetic Energy Situations.
From sciencing.com
How Do Energy and Potential Energy Apply to Everyday Life Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. energy is an important concept in physics that is used to understand large and small processes. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10. Kinetic Energy Situations.
From hyperphysics.phy-astr.gsu.edu
Energy Kinetic Energy Situations learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. Kinetic energy is the energy that any object with mass has simply because it is moving. energy is an important concept in physics that is used to understand large and small processes. This means that when the velocity of an object doubles, its kinetic. Kinetic Energy Situations.
From polarpedia.eu
energy Polarpedia Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. If an. Kinetic Energy Situations.
From ar.inspiredpencil.com
Energy Diagram For Kids Kinetic Energy Situations energy is an important concept in physics that is used to understand large and small processes. If an object is not moving, it. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. If work, which transfers energy,. learn. Kinetic Energy Situations.
From www.sciencelearn.org.nz
Potential and energy — Science Learning Hub Kinetic Energy Situations kinetic energy, form of energy that an object or a particle has by reason of its motion. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. kinetic energy depends on the velocity of the object squared. energy. Kinetic Energy Situations.
From www.dreamstime.com
and Potential Energy Explanation Labeled Vector Illustration Kinetic Energy Situations energy is an important concept in physics that is used to understand large and small processes. kinetic energy depends on the velocity of the object squared. kinetic energy, form of energy that an object or a particle has by reason of its motion. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10. Kinetic Energy Situations.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs Kinetic Energy Situations If work, which transfers energy,. If an object is not moving, it. This means that when the velocity of an object doubles, its kinetic energy quadruples. energy is an important concept in physics that is used to understand large and small processes. kinetic energy depends on the velocity of the object squared. Kinetic energy is the energy that. Kinetic Energy Situations.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs Kinetic Energy Situations learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. kinetic energy depends on the velocity of the object squared. If work, which transfers energy,. energy is an important concept in physics that is used to understand large and small processes. Kinetic energy is the energy that any object with mass has simply. Kinetic Energy Situations.
From www.teachoo.com
Energy Definition, Formula, Examples Teachoo Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. energy is an important concept in physics that is used to understand large and small processes. kinetic energy, form of energy that an object or a particle has by. Kinetic Energy Situations.
From db-excel.com
And Potential Energy Worksheet Pdf — Kinetic Energy Situations If an object is not moving, it. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. kinetic energy depends on the velocity of the object squared. Kinetic energy is the energy that any object with mass has simply because it is moving. If work, which transfers energy,. energy is an important concept. Kinetic Energy Situations.
From www.pinterest.com
How to Turn potential energy into energy DIY Potential Kinetic Energy Situations learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. If work, which transfers energy,. If an object is not moving, it. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. Kinetic energy is the. Kinetic Energy Situations.
From brainly.ph
Identify whether the object in the given situations possess potential Kinetic Energy Situations If work, which transfers energy,. This means that when the velocity of an object doubles, its kinetic energy quadruples. Kinetic energy is the energy that any object with mass has simply because it is moving. kinetic energy, form of energy that an object or a particle has by reason of its motion. If an object is not moving, it.. Kinetic Energy Situations.
From worldnewlive.com
How Do I Contact E Energy? Mastery Wiki Kinetic Energy Situations kinetic energy, form of energy that an object or a particle has by reason of its motion. This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21. Kinetic Energy Situations.
From www.jove.com
13505.jpg Kinetic Energy Situations learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. This means that when the velocity of an object doubles, its kinetic energy quadruples. If work, which transfers energy,. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10. Kinetic Energy Situations.
From www.slideserve.com
PPT Chapter 3 Alkenes PowerPoint Presentation, free download ID Kinetic Energy Situations learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. kinetic energy depends on the velocity of the object squared. If an object is not moving, it. This means that when the velocity of an object doubles, its kinetic energy quadruples. kinetic energy, form of energy that an object or a particle has. Kinetic Energy Situations.
From studylib.net
CHEMICAL Kinetic Energy Situations energy is an important concept in physics that is used to understand large and small processes. kinetic energy, form of energy that an object or a particle has by reason of its motion. Kinetic energy is the energy that any object with mass has simply because it is moving. If an object is not moving, it. If work,. Kinetic Energy Situations.
From azaltascip1.blogspot.com
Physical Science and Potential Energy Kinetic Energy Situations learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. Kinetic energy is the energy that any object with mass has simply because it is moving. energy is an important concept in physics that is used to understand large and small processes. kinetic energy depends on the velocity of the object squared. If. Kinetic Energy Situations.
From worldnewlive.com
What Is Energy And Its Formula? Mastery Wiki Kinetic Energy Situations Kinetic energy is the energy that any object with mass has simply because it is moving. learn about kinetic energy and its types, including translational, rotational, vibrational, thermal, and electrical. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,.. Kinetic Energy Situations.
From www.stuckincustoms.com
Energy Stuck in Customs Kinetic Energy Situations If work, which transfers energy,. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. kinetic energy depends on the velocity of the object squared. Kinetic energy is the energy that any object with mass has simply because it is. Kinetic Energy Situations.
From mrjeremycameron.com
Wednesday June 16th Education and Innovation Kinetic Energy Situations kinetic energy, form of energy that an object or a particle has by reason of its motion. Kinetic energy is the energy that any object with mass has simply because it is moving. If an object is not moving, it. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500. Kinetic Energy Situations.
From www.chegg.com
Solved In which of the following situations is the Kinetic Energy Situations kinetic energy, form of energy that an object or a particle has by reason of its motion. This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21. Kinetic Energy Situations.
From armfield.co.uk
EF2.4 Dynamics Potential and Energy Armfield Kinetic Energy Situations kinetic energy depends on the velocity of the object squared. This means that when the velocity of an object doubles, its kinetic energy quadruples. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m / s)2 = 6.02 × 10 − 21 j,. energy is an important concept. Kinetic Energy Situations.
From www.slideserve.com
PPT and Potential Energy PowerPoint Presentation, free Kinetic Energy Situations This means that when the velocity of an object doubles, its kinetic energy quadruples. If an object is not moving, it. kinetic energy, form of energy that an object or a particle has by reason of its motion. The average translational kinetic energy of a molecule is 1 2(29 u)(1.66 × 10 − 27 kg / u)(500 m /. Kinetic Energy Situations.