Soap Tails Chemistry . a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. However, as it is non. write an equation to represent the formation of a soap. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. Identify the structure of the fat required to produce a given soap. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left.
from chem.libretexts.org
soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. Identify the structure of the fat required to produce a given soap. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. write an equation to represent the formation of a soap. However, as it is non.
4.4 Solubility Chemistry LibreTexts
Soap Tails Chemistry Identify the structure of the fat required to produce a given soap. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Identify the structure of the fat required to produce a given soap. write an equation to represent the formation of a soap. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. However, as it is non.
From sciencenotes.org
Surfactant Definition and Examples Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. Identify the structure of the fat required to produce a given soap. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. write. Soap Tails Chemistry.
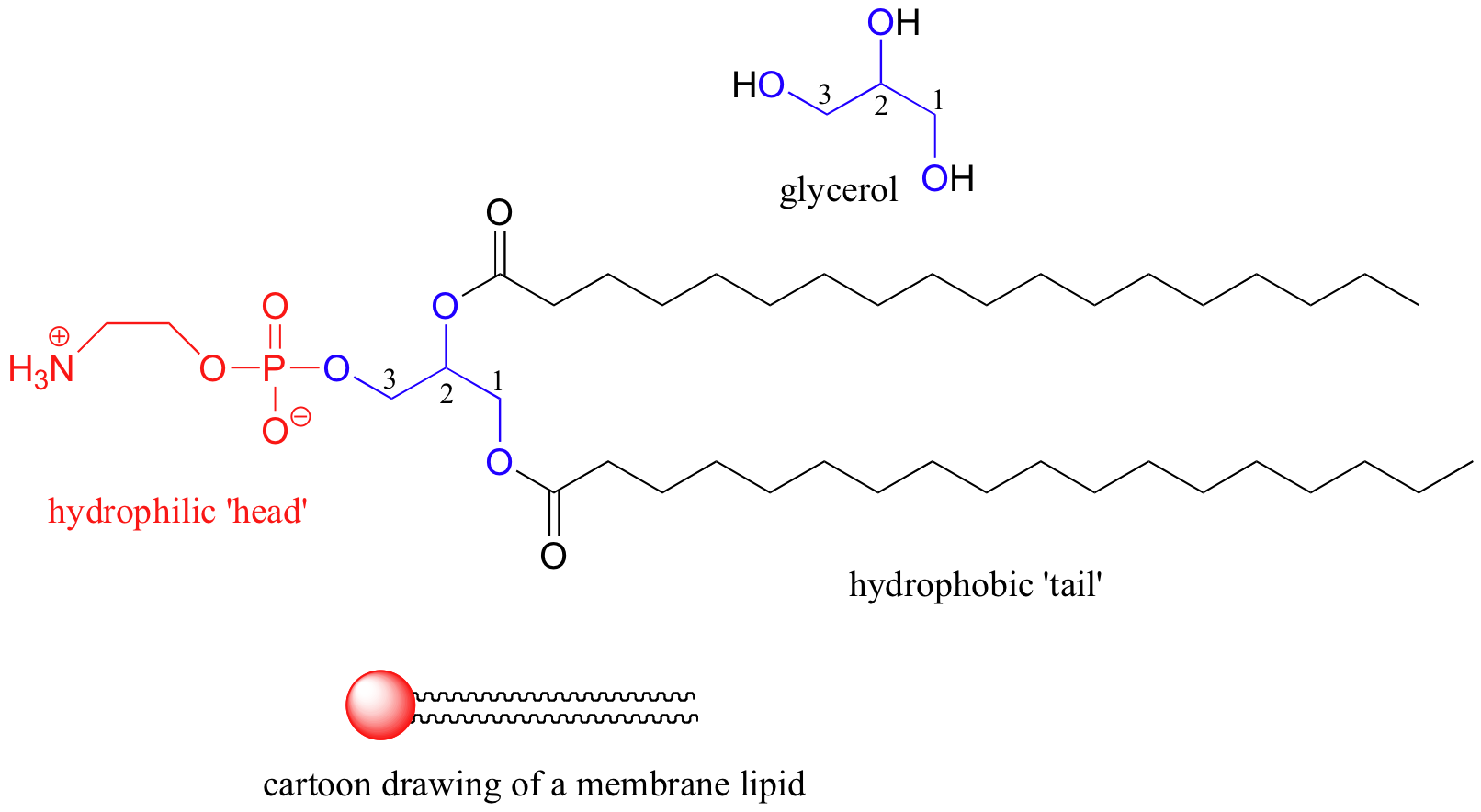
From oerpub.github.io
This diagram shows the structure of a phospholipid. The hydrophilic Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. write an equation to represent the formation of a soap. However, as it is non. Identify the structure of the fat required to produce a given soap. a drop or two of soap in. Soap Tails Chemistry.
From www.mdpi.com
Chemistry Free FullText Head vs. Tail SquaramideNaphthalimide Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. write an equation to represent the formation of a soap. However,. Soap Tails Chemistry.
From www.numerade.com
SOLVED Homework 12 Chapter 15 Value 1.81 points Out of 3 attempts Soap Tails Chemistry However, as it is non. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. Identify the structure of the fat required to produce. Soap Tails Chemistry.
From www.chembook.co.uk
chembook.co.uk CHEMISTRY IN PERSPECTIVE FOR BORED AND CONFUSED SENIOR Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. write an equation to represent the formation of a soap.. Soap Tails Chemistry.
From chem.libretexts.org
4.4 Solubility Chemistry LibreTexts Soap Tails Chemistry However, as it is non. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap and detergent, substances that, when dissolved. Soap Tails Chemistry.
From cosmosmagazine.com
The chemistry of soap Soap Tails Chemistry soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. However, as it is non. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in. Soap Tails Chemistry.
From aliceinchemiland.blogspot.my
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Soap Tails Chemistry Identify the structure of the fat required to produce a given soap. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. write an equation to represent the formation of a soap. soap and detergent, substances that, when dissolved in water, possess the ability. Soap Tails Chemistry.
From chem.libretexts.org
Chapter 13.6 Aggregate Particles Chemistry LibreTexts Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. Identify the structure of the fat required to produce a given soap. write an equation to represent the formation of a soap. However, as it is non. soap and detergent, substances that, when dissolved. Soap Tails Chemistry.
From cosmosmagazine.com
The chemistry of soap Soap Tails Chemistry Identify the structure of the fat required to produce a given soap. However, as it is non. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in. Soap Tails Chemistry.
From www.aplustopper.com
Explain the Cleansing Action Of Soaps and Detergents A Plus Topper Soap Tails Chemistry soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. Identify the structure of the fat required to produce a given soap. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left.. Soap Tails Chemistry.
From my-chem-assignment.blogspot.com
Chemistry Assignment Soap and Detergent Soap Tails Chemistry However, as it is non. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. Identify the structure of the fat required. Soap Tails Chemistry.
From www.theodysseyonline.com
How Does Soap Work? Soap Tails Chemistry soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. write an equation to represent the formation of a soap. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left.. Soap Tails Chemistry.
From chem.libretexts.org
17.2 Fats and Oils Chemistry LibreTexts Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap molecules have a hybrid structure, with a hydrophilic head. Soap Tails Chemistry.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. soap is a salt of an alkali metal, such as sodium. Soap Tails Chemistry.
From www.pinterest.com
tails. Handmade soaps, Handmade soap, Handmade Soap Tails Chemistry Identify the structure of the fat required to produce a given soap. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. soap. Soap Tails Chemistry.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. However, as it is non. write an equation to represent the formation of a soap. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that. Soap Tails Chemistry.
From xed.ch
What Soap Really Does Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. soap molecules have a hybrid structure, with a hydrophilic. Soap Tails Chemistry.
From cosmosmagazine.com
The chemistry of soap Soap Tails Chemistry soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. write an equation to represent the formation of a soap. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. However, as. Soap Tails Chemistry.
From www.youtube.com
Chemistry Tails YouTube Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. However, as it is non. write an equation to represent the. Soap Tails Chemistry.
From www.kearney.com
Unlocking the hidden value of chemicals tail spend Kearney Soap Tails Chemistry soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. Identify the structure of the fat required to produce a given soap. . Soap Tails Chemistry.
From www.fanart-central.net
Chemistry Tails by Silverfeather Fanart Central Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. a drop or two of soap in water forms. Soap Tails Chemistry.
From www.pinterest.co.uk
Hand washing with soap vector illustration. Educational explanation Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. Identify the structure of the fat required to produce a given soap.. Soap Tails Chemistry.
From lestwinsonline.com
Top 125 + How soap works animation Soap Tails Chemistry However, as it is non. Identify the structure of the fat required to produce a given soap. write an equation to represent the formation of a soap. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap molecules have a hybrid structure, with a. Soap Tails Chemistry.
From cbse.myindialist.com
Chemistry X Carbon and its Compounds SOAPS AND DETERGENTS CBSE Soap Tails Chemistry a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. soap is a salt of an alkali metal, such as sodium. Soap Tails Chemistry.
From www.science.org.au
Hand sanitiser or soap making an informed choice for COVID19 Curious Soap Tails Chemistry soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids it. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Identify the structure of the fat required to produce a given soap. soap and. Soap Tails Chemistry.
From www.youtube.com
[5.1] Cleansing action of soap YouTube Soap Tails Chemistry Identify the structure of the fat required to produce a given soap. However, as it is non. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. a drop or two of soap in water forms a monolayer on the water surface as. Soap Tails Chemistry.
From www.pinterest.com
Mermaid Tail Soap Handmade soap gifts, Mermaid soap, Handmade artisan Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. However, as it is non. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap and detergent, substances that, when dissolved. Soap Tails Chemistry.
From chemistry.about.com
What Is Soap Made of and How Does It Clean? Soap Tails Chemistry However, as it is non. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. Identify the structure of the. Soap Tails Chemistry.
From www.themacbath.com
Back to Basics What Is Soap and How Does It Work? — The MacBath Soap Tails Chemistry Identify the structure of the fat required to produce a given soap. a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. . Soap Tails Chemistry.
From byjus.com
Cleansing action of soap is due to the formation of micelles. Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. However, as it is non. Identify the structure of the fat required to produce a given soap. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “. Soap Tails Chemistry.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap Tails Chemistry write an equation to represent the formation of a soap. soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. Identify the structure of the fat required to produce a given soap. soap is a salt of an alkali metal, such as sodium or. Soap Tails Chemistry.
From profesionalmutadayyin.blogspot.com
Analysing Detergent Prof.Mutadayyin Soap Tails Chemistry write an equation to represent the formation of a soap. Identify the structure of the fat required to produce a given soap. However, as it is non. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. a drop or two of soap in water. Soap Tails Chemistry.
From courses.lumenlearning.com
Components and Structure OpenStax Biology 2e Soap Tails Chemistry soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown in the graphics on the left. write an equation to represent the formation of a. Soap Tails Chemistry.
From chemwiki.ucdavis.edu
13.6 Aggregate Particles in Aqueous Solution Chemwiki Soap Tails Chemistry a drop or two of soap in water forms a monolayer on the water surface as shown in the graphics on the left. write an equation to represent the formation of a soap. However, as it is non. a drop or two of soap in water forms a monolayer (figure \(\pageindex{5}\)) on the water surface as shown. Soap Tails Chemistry.