Master Record Number Definition . What is a device master record (dmr)? When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as A device master record is a collection of every document needed to manufacture, package, and. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. The device master record is a regulatory requirement for all medical device companies. What is a device master record? What is a device master record? Dmr is called the medical device register and corresponds to japanese product standards.
from www.tutorialspoint.com
Dmr is called the medical device register and corresponds to japanese product standards. What is a device master record (dmr)? It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. What is a device master record? A device master record is a collection of every document needed to manufacture, package, and. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as The device master record is a regulatory requirement for all medical device companies. What is a device master record? A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to.
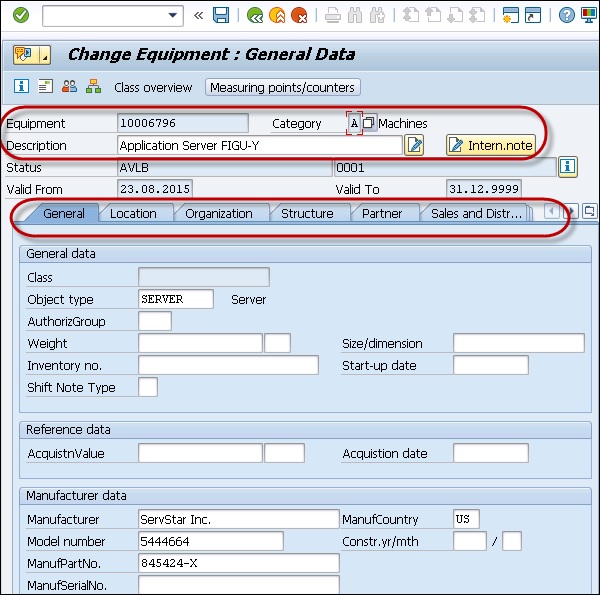
SAP PM Equipment Master Record
Master Record Number Definition It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. Dmr is called the medical device register and corresponds to japanese product standards. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. What is a device master record (dmr)? The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. What is a device master record? What is a device master record? A device master record is a collection of every document needed to manufacture, package, and. When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as
From imc.3jpharmainc.com
Difference Between master Formulation Record And Compounding Record For Master Record Number Definition The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental. Master Record Number Definition.
From posts.specterops.io
Hostbased Threat Modeling & Indicator Design Posts By SpecterOps Master Record Number Definition It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record is a collection of every document needed to manufacture, package, and. Dmr is called the medical device register and corresponds to japanese product standards. When it boils. Master Record Number Definition.
From www.slideserve.com
PPT Records Retention PowerPoint Presentation, free download ID504360 Master Record Number Definition What is a device master record (dmr)? A device master record is a collection of every document needed to manufacture, package, and. What is a device master record? A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Dmr is called the medical device register and corresponds to japanese product standards. The. Master Record Number Definition.
From dokumen.tips
(PDF) Asset Master Record Louisiana Presentation.pdf · Asset Master Master Record Number Definition A device master record is a collection of every document needed to manufacture, package, and. What is a device master record (dmr)? A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of. Master Record Number Definition.
From www.scribd.com
Master Formula Record Sample PDF PDF Master Record Number Definition A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Dmr is called the medical device register and corresponds to japanese product standards. What is a device master record? What is a device master record (dmr)? It is a repository of all essential information about your company’s medical devices and includes design. Master Record Number Definition.
From www.slideshare.net
Basics of records management Master Record Number Definition It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. What is a device master record? A device master record is a collection of every document needed to manufacture, package, and. When it boils down to it, investing in a quality. Master Record Number Definition.
From stusupplychain.com
What is IOR? Importer of Record Definition Master Record Number Definition What is a device master record? The device master record is a regulatory requirement for all medical device companies. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. Dmr is called the medical device register and corresponds to japanese product standards. A device. Master Record Number Definition.
From www.slideserve.com
PPT Intro to IDMS PowerPoint Presentation, free download ID5200443 Master Record Number Definition A device master record is a collection of every document needed to manufacture, package, and. Dmr is called the medical device register and corresponds to japanese product standards. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. The device master record is a. Master Record Number Definition.
From www.tutorialspoint.com
SAP PM Equipment Master Record Master Record Number Definition A device master record is a collection of every document needed to manufacture, package, and. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. Dmr is called the medical device register and corresponds to japanese product standards. What is a device master record. Master Record Number Definition.
From dokopa.com
マスターナンバーの遊び方とコツ どこパ公式サイト Master Record Number Definition A device master record is a collection of every document needed to manufacture, package, and. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Dmr is called the medical device register and corresponds to japanese product standards. The device master record (dmr) is established under title 21 of the cfr, part. Master Record Number Definition.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Record Number Definition What is a device master record? When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a. Master Record Number Definition.
From videos.to-increase.com
Create & Manage Master Records in MDM Studio — Part 1(2) ToIncrease Master Record Number Definition When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as Dmr is called the medical device register and corresponds to japanese product standards. What is a device master record? The device master record (dmr) is established under. Master Record Number Definition.
From www.youtube.com
S/4 HANA Validation For Asset Master Record FICO YouTube Master Record Number Definition It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. What is a device master record (dmr)? What is a device master record?. Master Record Number Definition.
From www.scribd.com
Master Record Sheet Lighthouse Christian Academy Account Name Account Master Record Number Definition What is a device master record? A device master record is a collection of every document needed to manufacture, package, and. The device master record is a regulatory requirement for all medical device companies. When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping. Master Record Number Definition.
From fyosjnvir.blob.core.windows.net
Device Master Record Definition Fda at Danny Forsythe blog Master Record Number Definition The device master record is a regulatory requirement for all medical device companies. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. What is a device master record (dmr)? When it boils down to it, investing in a quality management system (qms) is. Master Record Number Definition.
From www.tutorialkart.com
Create Customer Master Record in SAP (Customer Master Data) Master Record Number Definition Dmr is called the medical device register and corresponds to japanese product standards. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records.. Master Record Number Definition.
From www.youtube.com
Golden Record in Master Data Management (MDM) The Data Governor YouTube Master Record Number Definition The device master record is a regulatory requirement for all medical device companies. When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as Dmr is called the medical device register and corresponds to japanese product standards. What. Master Record Number Definition.
From olaz1-wpmk3.azurewebsites.net
Add a Record Index and Count to your document OL® Learn Master Record Number Definition The device master record is a regulatory requirement for all medical device companies. A device master record is a collection of every document needed to manufacture, package, and. Dmr is called the medical device register and corresponds to japanese product standards. What is a device master record? The device master record (dmr) is established under title 21 of the cfr,. Master Record Number Definition.
From www.youtube.com
Preparing a Device Master Record (DMR) YouTube Master Record Number Definition When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. The. Master Record Number Definition.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Master Record Number Definition What is a device master record? When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality. Master Record Number Definition.
From citizenside.com
Master Boot Record Definition (MBR, Sector Zero) CitizenSide Master Record Number Definition Dmr is called the medical device register and corresponds to japanese product standards. When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as It is a repository of all essential information about your company’s medical devices and. Master Record Number Definition.
From community.sap.com
Asset Accounting How are the Asset Master Record... SAP Community Master Record Number Definition What is a device master record? A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. The device master record is a regulatory requirement for all. Master Record Number Definition.
From uaeexpatriates.com
5 Steps to Create an MRN Number in Dubai UAE Expatriates Master Record Number Definition When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings,. Master Record Number Definition.
From psofthelp.blogspot.com
PeopleSoft Help Record Definitions in PeopleTools Master Record Number Definition What is a device master record (dmr)? It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. The device master record is a. Master Record Number Definition.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Record Number Definition What is a device master record? When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive. Master Record Number Definition.
From www.biobostonconsulting.com
Crucial Components of the Device Master Record (DMR) for Medical Device Master Record Number Definition What is a device master record? It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record is a collection of every document needed to manufacture, package, and. Dmr is called the medical device register and corresponds to. Master Record Number Definition.
From www.salesforcehacker.com
Salesforce Hacker Master Record Types with Permission Sets Master Record Number Definition When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to ensure the safekeeping of your device master record — as A device master record is a collection of every document needed to manufacture, package, and. It is a repository of all essential information about your company’s medical. Master Record Number Definition.
From www.technia.co.jp
What is a Device Master Record? TECHNIA (Japan) Master Record Number Definition Dmr is called the medical device register and corresponds to japanese product standards. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element of quality systems regulations for medical devices. It is a. Master Record Number Definition.
From www.researchgate.net
FAA Form 5010Airport Master Record (example). Download Scientific Master Record Number Definition Dmr is called the medical device register and corresponds to japanese product standards. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. What is a device master record? A device master record (dmr) is a comprehensive compilation of all the. Master Record Number Definition.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Record Number Definition A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. What is a device master record? The device master record is a regulatory requirement for all medical device companies. When it boils down to it, investing in a quality management system (qms) is the single most important thing you can do to. Master Record Number Definition.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Master Record Number Definition What is a device master record? A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. What is a device master record (dmr)? It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records.. Master Record Number Definition.
From help.nfc.usda.gov
Delete/Restore Master Record (DM) or Delete/Restore Individual Position Master Record Number Definition What is a device master record? A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. The device master record is a regulatory requirement for all medical device companies. Dmr is called the medical device register and corresponds to japanese product standards. A device master record is a collection of every document. Master Record Number Definition.
From www.scaler.com
What is the Master Boot Record (MBR)? Scaler Topics Master Record Number Definition What is a device master record (dmr)? What is a device master record? Dmr is called the medical device register and corresponds to japanese product standards. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. The device master record (dmr). Master Record Number Definition.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Master Record Number Definition A device master record is a collection of every document needed to manufacture, package, and. What is a device master record? What is a device master record? Dmr is called the medical device register and corresponds to japanese product standards. What is a device master record (dmr)? When it boils down to it, investing in a quality management system (qms). Master Record Number Definition.
From www.slideshare.net
SBS102 DocumentvsRecords Master Record Number Definition What is a device master record? Dmr is called the medical device register and corresponds to japanese product standards. A device master record is a collection of every document needed to manufacture, package, and. What is a device master record (dmr)? The device master record (dmr) is established under title 21 of the cfr, part 820, as a fundamental element. Master Record Number Definition.