Standard Hydrogen Electrode Questions . It has a cell potential of 0.00v , measured. (a) state the substances and. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. The standard hydrogen electrode is often. The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? Examples of using the standard hydrogen electrode to determine reduction potentials are given. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Hydrogen gas in equilibrium with h + ions of.
from www.chegg.com
Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. standard electrode potentials are measured by comparison with the standard hydrogen electrode. The standard hydrogen electrode is often. What is a standard hydrogen electrode? It has a cell potential of 0.00v , measured. (a) state the substances and. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. Hydrogen gas in equilibrium with h + ions of.
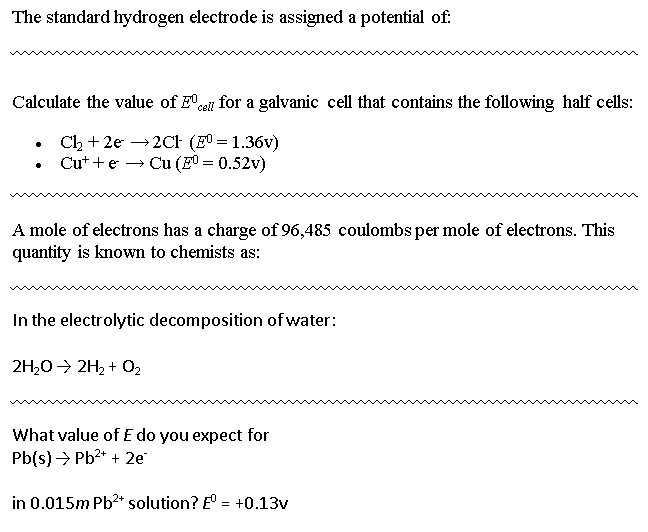
Solved The standard hydrogen electrode is assigned a
Standard Hydrogen Electrode Questions draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. Hydrogen gas in equilibrium with h + ions of. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. standard electrode potentials are measured by comparison with the standard hydrogen electrode. (a) state the substances and. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Examples of using the standard hydrogen electrode to determine reduction potentials are given. It has a cell potential of 0.00v , measured. What is a standard hydrogen electrode? The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Questions The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? standard electrode potentials are measured by comparison with the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Hydrogen gas in equilibrium with h + ions of. the standard hydrogen electrode is the standard measurement of. Standard Hydrogen Electrode Questions.
From www.chegg.com
Solved to what pH should you adjust a standard hydrogen Standard Hydrogen Electrode Questions the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. standard electrode potentials are measured by comparison with the standard hydrogen electrode. The standard hydrogen electrode is often.. Standard Hydrogen Electrode Questions.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Questions a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? the standard hydrogen electrode is the standard measurement of electrode potential for the. Standard Hydrogen Electrode Questions.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry Standard Hydrogen Electrode Questions draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. What is a standard hydrogen electrode? The structure of the standard hydrogen electrode is described. Hydrogen. Standard Hydrogen Electrode Questions.
From brainly.in
write the symbolic representation of standard hydrogen electrode Standard Hydrogen Electrode Questions draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. (a) state the substances and. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often. Examples of using the standard hydrogen electrode to determine reduction potentials are given. a hydrogen electrode in which pressure of hydrogen. Standard Hydrogen Electrode Questions.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Standard Hydrogen Electrode Questions Examples of using the standard hydrogen electrode to determine reduction potentials are given. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is. Standard Hydrogen Electrode Questions.
From www.doubtnut.com
Describe the construction and working of the standard hydrogen electr Standard Hydrogen Electrode Questions What is a standard hydrogen electrode? The standard hydrogen electrode is often. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. It has a cell potential of 0.00v , measured. (a) state the substances and. standard electrode. Standard Hydrogen Electrode Questions.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Questions a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. It has a cell potential of 0.00v , measured. The structure of the standard hydrogen electrode is described. draw clearly labelled diagrams to show the components and reagents,. Standard Hydrogen Electrode Questions.
From www.chegg.com
Solved A standard hydrogen electrode (SHE) is shown in the Standard Hydrogen Electrode Questions the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. Examples of using the standard hydrogen electrode to determine reduction. Standard Hydrogen Electrode Questions.
From www.youtube.com
Standard Hydrogen Electrode Electrochemistry Chemistry Class 12 Standard Hydrogen Electrode Questions The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. Hydrogen gas in equilibrium with h + ions of. (a) state the substances and.. Standard Hydrogen Electrode Questions.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Standard Hydrogen Electrode Questions The standard hydrogen electrode is often. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. The structure of the standard hydrogen electrode is described. It has a cell potential of 0.00v , measured. (a) state the substances and.. Standard Hydrogen Electrode Questions.
From www.numerade.com
SOLVED In the following cell, A is a standard Sn2tISn electrode Standard Hydrogen Electrode Questions What is a standard hydrogen electrode? the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. a hydrogen electrode in which pressure of hydrogen. Standard Hydrogen Electrode Questions.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Questions The standard hydrogen electrode is often. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the. Standard Hydrogen Electrode Questions.
From www.youtube.com
Class 12 Electrochemistry / How electrode potential is measured by Standard Hydrogen Electrode Questions The structure of the standard hydrogen electrode is described. It has a cell potential of 0.00v , measured. (a) state the substances and. What is a standard hydrogen electrode? standard electrode potentials are measured by comparison with the standard hydrogen electrode. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox. Standard Hydrogen Electrode Questions.
From www.numerade.com
SOLVED The standard hydrogen electrode Select the correct answer Standard Hydrogen Electrode Questions What is a standard hydrogen electrode? standard electrode potentials are measured by comparison with the standard hydrogen electrode. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Examples of using the standard hydrogen electrode to determine reduction potentials are given. a hydrogen electrode in which pressure of hydrogen. Standard Hydrogen Electrode Questions.
From www.numerade.com
SOLVEDDraw a diagram of a standard hydrogen electrode and describe how Standard Hydrogen Electrode Questions The structure of the standard hydrogen electrode is described. Hydrogen gas in equilibrium with h + ions of. What is a standard hydrogen electrode? draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. It has a cell potential of 0.00v , measured. a hydrogen electrode in which pressure of hydrogen. Standard Hydrogen Electrode Questions.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Questions What is a standard hydrogen electrode? The structure of the standard hydrogen electrode is described. (a) state the substances and. The standard hydrogen electrode is often. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Hydrogen gas in equilibrium with h + ions of. It has a cell potential of 0.00v , measured. the standard. Standard Hydrogen Electrode Questions.
From www.numerade.com
SOLVEDDraw a diagram of a standard hydrogen electrode and describe how Standard Hydrogen Electrode Questions Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. standard electrode potentials are measured by comparison with the standard hydrogen electrode. What is a standard hydrogen electrode? a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of. Standard Hydrogen Electrode Questions.
From exoyfxrvd.blob.core.windows.net
Standard Hydrogen Electrode Nernst Equation at Silva Upchurch blog Standard Hydrogen Electrode Questions Hydrogen gas in equilibrium with h + ions of. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. Examples of using the standard hydrogen electrode to determine reduction potentials are given. standard electrode potentials are measured by comparison with the standard hydrogen electrode. The standard hydrogen electrode is often. What. Standard Hydrogen Electrode Questions.
From www.youtube.com
The Standard hydrogen electrode (SHE) YouTube Standard Hydrogen Electrode Questions a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Hydrogen gas in equilibrium with h + ions of. (a). Standard Hydrogen Electrode Questions.
From www.chegg.com
Solved Match the following labels with the appropriate Standard Hydrogen Electrode Questions What is a standard hydrogen electrode? It has a cell potential of 0.00v , measured. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Hydrogen gas in equilibrium with h + ions of. (a) state the substances and. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell.. Standard Hydrogen Electrode Questions.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Questions Hydrogen gas in equilibrium with h + ions of. The structure of the standard hydrogen electrode is described. standard electrode potentials are measured by comparison with the standard hydrogen electrode. The standard hydrogen electrode is often. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. It has a cell. Standard Hydrogen Electrode Questions.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Questions draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode is often. (a) state the substances and. The structure of the standard. Standard Hydrogen Electrode Questions.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Questions The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? (a) state the substances and. Examples of using the standard hydrogen electrode to determine reduction potentials are given. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Hydrogen gas in equilibrium with h + ions of. The standard hydrogen electrode is. Standard Hydrogen Electrode Questions.
From www.chegg.com
Solved Which of the following statement is true about the Standard Hydrogen Electrode Questions What is a standard hydrogen electrode? Hydrogen gas in equilibrium with h + ions of. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. standard electrode potentials are measured by comparison with the standard hydrogen electrode. the standard hydrogen electrode is the standard measurement of electrode potential for the. Standard Hydrogen Electrode Questions.
From askfilo.com
Standard hydrogen electrode (SHE) It is used as reference electrode who.. Standard Hydrogen Electrode Questions standard electrode potentials are measured by comparison with the standard hydrogen electrode. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. The structure of the standard hydrogen electrode is described. (a) state the substances and. The standard hydrogen electrode is often. Examples of using the standard hydrogen electrode to determine. Standard Hydrogen Electrode Questions.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Questions It has a cell potential of 0.00v , measured. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. (a) state the substances and. The structure. Standard Hydrogen Electrode Questions.
From www.chegg.com
Solved what would the potential of a standard hydrogen Standard Hydrogen Electrode Questions the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Hydrogen gas in equilibrium with h + ions of. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode is often. It has a cell potential of 0.00v , measured. The structure of the. Standard Hydrogen Electrode Questions.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Questions a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and the concentration of h + ions in the solution is 1m, is called a standard. The standard hydrogen electrode is often. the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The structure of the. Standard Hydrogen Electrode Questions.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube Standard Hydrogen Electrode Questions the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. (a) state the substances and. standard electrode potentials are measured by comparison with the standard hydrogen electrode. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often. Hydrogen gas in equilibrium with h + ions. Standard Hydrogen Electrode Questions.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Standard Hydrogen Electrode Questions draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. Examples of using the standard hydrogen electrode to determine reduction potentials are given. It has a cell potential of 0.00v , measured. (a) state the substances and. Hydrogen gas in equilibrium with h + ions of. the standard hydrogen electrode is. Standard Hydrogen Electrode Questions.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Questions draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. What is a standard hydrogen electrode? the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Hydrogen gas in equilibrium with h + ions of. Examples of using the standard hydrogen electrode to. Standard Hydrogen Electrode Questions.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Standard Hydrogen Electrode Questions the standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The standard hydrogen electrode is often. (a) state the substances and. standard electrode potentials are measured by comparison with the standard hydrogen electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Hydrogen gas in equilibrium. Standard Hydrogen Electrode Questions.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Questions The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. (a) state the substances and. The standard hydrogen electrode is often. a hydrogen electrode in which pressure of hydrogen gas is maintained at 1 atm and. Standard Hydrogen Electrode Questions.
From www.chegg.com
Solved The standard hydrogen electrode is assigned a Standard Hydrogen Electrode Questions The standard hydrogen electrode is often. Hydrogen gas in equilibrium with h + ions of. The structure of the standard hydrogen electrode is described. standard electrode potentials are measured by comparison with the standard hydrogen electrode. draw clearly labelled diagrams to show the components and reagents, including their concentrations, in this electrochemical cell. a hydrogen electrode in. Standard Hydrogen Electrode Questions.