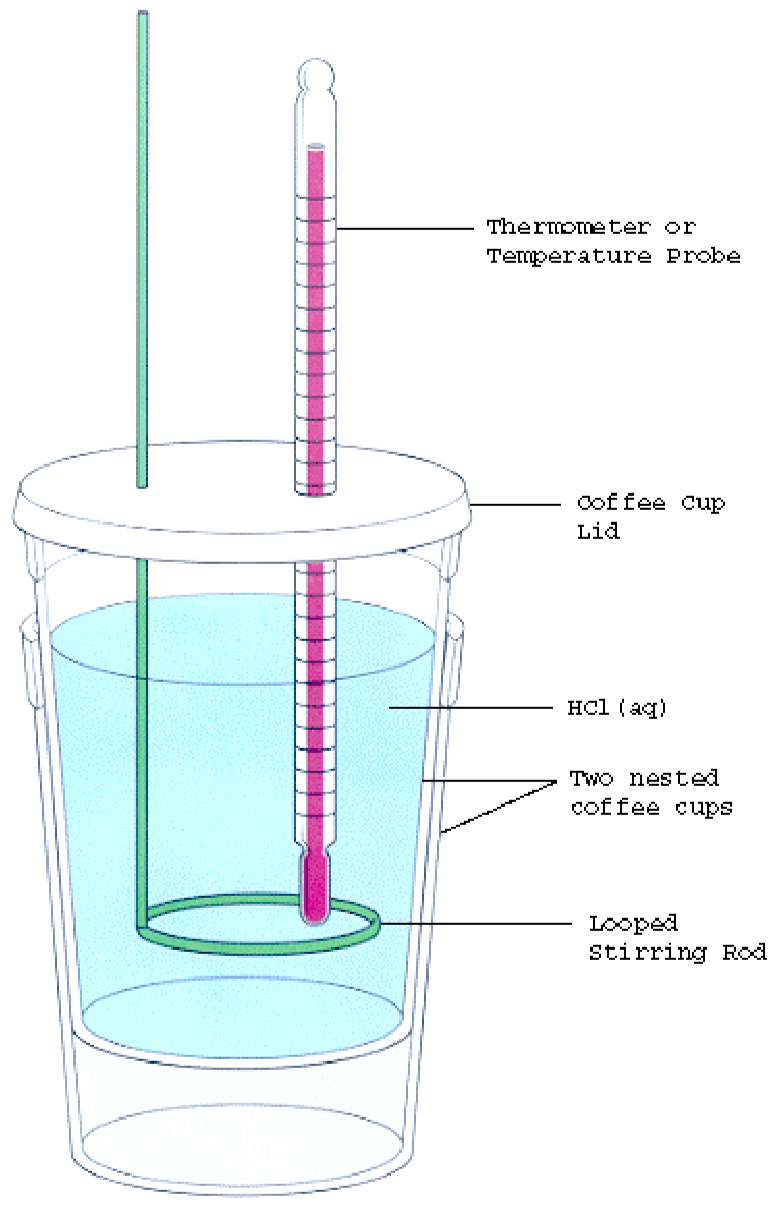
Calorimetry Experiment Bbc Bitesize . Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Learn about the specific heat capacity practical in this podcast. A method which allows enthalpies of a reaction to be determined by experiment. Listen to the full series on bbc sounds. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. To measure the specific heat capacity. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. What equation would you use to. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in.
from chem.libretexts.org
In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Listen to the full series on bbc sounds. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. A method which allows enthalpies of a reaction to be determined by experiment. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Learn about the specific heat capacity practical in this podcast. To measure the specific heat capacity. What equation would you use to. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Calorimetry Experiment Bbc Bitesize Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. What equation would you use to. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. A method which allows enthalpies of a reaction to be determined by experiment. Listen to the full series on bbc sounds. Learn about the specific heat capacity practical in this podcast. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. To measure the specific heat capacity. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Calorimetry Experiment Bbc Bitesize In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples What equation would you use to. Revise your understanding of enzymes, substrates, lock and key theory and. Calorimetry Experiment Bbc Bitesize.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Experiment Bbc Bitesize Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. To measure the specific heat capacity. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. In this experiment you will heat a known mass of a metal to a known temperature and. Calorimetry Experiment Bbc Bitesize.
From youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Experiment Bbc Bitesize Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples A method which allows enthalpies of a reaction to be determined by experiment. What equation would you use to. Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving. Calorimetry Experiment Bbc Bitesize.
From pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Experiment Bbc Bitesize Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. To measure the specific. Calorimetry Experiment Bbc Bitesize.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimetry Experiment Bbc Bitesize A method which allows enthalpies of a reaction to be determined by experiment. Listen to the full series on bbc sounds. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts. Calorimetry Experiment Bbc Bitesize.
From www.studocu.com
Calorimetry Gizmo Student Exploration Calorimetry Lab Gizmo Warmup Calorimetry Experiment Bbc Bitesize To measure the specific heat capacity. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. What equation would you use to. Listen to the full series on bbc sounds. Learn about the specific heat capacity practical in this podcast. In this experiment you will heat a known mass of. Calorimetry Experiment Bbc Bitesize.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.3 Calorimetry翰林国际教育 Calorimetry Experiment Bbc Bitesize A method which allows enthalpies of a reaction to be determined by experiment. Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Listen to the full series on bbc sounds. To measure the specific heat capacity. In this experiment you will heat. Calorimetry Experiment Bbc Bitesize.
From www.bbc.co.uk
Calorimetry Enzymes Edexcel GCSE Biology (Single Science Calorimetry Experiment Bbc Bitesize Listen to the full series on bbc sounds. What equation would you use to. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Different food samples can be. Calorimetry Experiment Bbc Bitesize.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Calorimetry Experiment Bbc Bitesize Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples To measure the specific heat capacity. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Listen to the full series on bbc sounds. What equation would you use to.. Calorimetry Experiment Bbc Bitesize.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Calorimetry Experiment Bbc Bitesize 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Listen to the full series on bbc sounds. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Learn about the specific heat capacity practical in this podcast. Different food samples can. Calorimetry Experiment Bbc Bitesize.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Experiment Bbc Bitesize Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. A method which allows enthalpies of a reaction to be determined by experiment. What equation would you use to. Learn about the specific heat capacity practical in this podcast. To measure the specific heat capacity. Simple calorimetry experiments can be. Calorimetry Experiment Bbc Bitesize.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Experiment Bbc Bitesize Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it. Calorimetry Experiment Bbc Bitesize.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimetry Experiment Bbc Bitesize Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Learn about the specific heat capacity practical in this podcast. A method which allows enthalpies of a reaction to be determined by experiment. Listen to the full series on bbc sounds. Revise your understanding of enzymes, substrates, lock and key theory. Calorimetry Experiment Bbc Bitesize.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Experiment Bbc Bitesize Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Listen to the full series on bbc sounds. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. To. Calorimetry Experiment Bbc Bitesize.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Experiment Bbc Bitesize A method which allows enthalpies of a reaction to be determined by experiment. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Listen to the full series on bbc sounds. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. To. Calorimetry Experiment Bbc Bitesize.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Calorimetry Experiment Bbc Bitesize In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. What equation would you use to. Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. To measure the. Calorimetry Experiment Bbc Bitesize.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Calorimetry Experiment Bbc Bitesize 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples What equation would you use to. A method which allows enthalpies of a reaction to be determined by experiment. Listen to the full series. Calorimetry Experiment Bbc Bitesize.
From www.researchgate.net
Temperature measurements during a typical calorimetry experiment Calorimetry Experiment Bbc Bitesize Learn about the specific heat capacity practical in this podcast. What equation would you use to. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Listen to the full. Calorimetry Experiment Bbc Bitesize.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Bbc Bitesize 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving. Calorimetry Experiment Bbc Bitesize.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Calorimetry Experiment Bbc Bitesize Learn about the specific heat capacity practical in this podcast. A method which allows enthalpies of a reaction to be determined by experiment. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Simple calorimetry experiments can be used to calculate the heat energy transferred during. Calorimetry Experiment Bbc Bitesize.
From www.youtube.com
Thermal Properties of Matter (Calorimetry, Calorimeter) part 3 Calorimetry Experiment Bbc Bitesize Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. To measure the specific heat capacity. In this experiment you will heat a. Calorimetry Experiment Bbc Bitesize.
From www.pinterest.com
Calorimetry Calorimetry Experiment Bbc Bitesize Listen to the full series on bbc sounds. To measure the specific heat capacity. Learn about the specific heat capacity practical in this podcast. What equation would you use to. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Different food samples can be burned. Calorimetry Experiment Bbc Bitesize.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiment Bbc Bitesize Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. What equation would you use to. A method which allows enthalpies of a reaction to be determined by experiment. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on.. Calorimetry Experiment Bbc Bitesize.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Experiment Bbc Bitesize Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. A method which allows enthalpies of a reaction to be determined by experiment. Revise your understanding of enzymes, substrates, lock and key. Calorimetry Experiment Bbc Bitesize.
From 2012books.lardbucket.org
Calorimetry Calorimetry Experiment Bbc Bitesize Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. Learn about the specific heat capacity practical in this podcast. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples What equation would you use to. 3.2 describe simple calorimetry experiments. Calorimetry Experiment Bbc Bitesize.
From www.studocu.com
2 Solution Calorimetry Experiment 2 Solution Calorimetry Prepared by Calorimetry Experiment Bbc Bitesize In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Listen to the full series on bbc sounds. Learn about the specific heat capacity practical in this podcast. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the. Calorimetry Experiment Bbc Bitesize.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Experiment Bbc Bitesize Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples A method which allows enthalpies of a reaction to be determined by experiment. Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Simple. Calorimetry Experiment Bbc Bitesize.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry Experiment Bbc Bitesize Learn about the specific heat capacity practical in this podcast. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. 3.2 describe simple calorimetry experiments for. Calorimetry Experiment Bbc Bitesize.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Experiment Bbc Bitesize A method which allows enthalpies of a reaction to be determined by experiment. Listen to the full series on bbc sounds. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. To measure the specific heat capacity. Simple calorimetry experiments can be used to calculate the. Calorimetry Experiment Bbc Bitesize.
From www.scribd.com
Calorimetry Experiment PDF Heat Enthalpy Calorimetry Experiment Bbc Bitesize 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and ph on. Listen to the full series on bbc sounds. Different food samples can be burned in a simple calorimetry experiment to compare the energy. Calorimetry Experiment Bbc Bitesize.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Experiment Bbc Bitesize 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Learn about the specific heat capacity practical in this podcast. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples What equation would you use to. Revise your understanding of enzymes, substrates, lock and. Calorimetry Experiment Bbc Bitesize.
From www.youtube.com
Introduction to Calorimetry YouTube Calorimetry Experiment Bbc Bitesize Listen to the full series on bbc sounds. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving. Calorimetry Experiment Bbc Bitesize.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimetry Experiment Bbc Bitesize Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. To measure the specific heat capacity. Listen to the full series on bbc sounds. Learn about the specific heat capacity practical in. Calorimetry Experiment Bbc Bitesize.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Experiment Bbc Bitesize In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. A method which allows enthalpies of a reaction to be determined by experiment. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. To measure. Calorimetry Experiment Bbc Bitesize.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry Experiment Bbc Bitesize Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. To measure the specific heat capacity. Learn about the specific heat capacity practical in this podcast. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Revise your understanding of enzymes, substrates,. Calorimetry Experiment Bbc Bitesize.