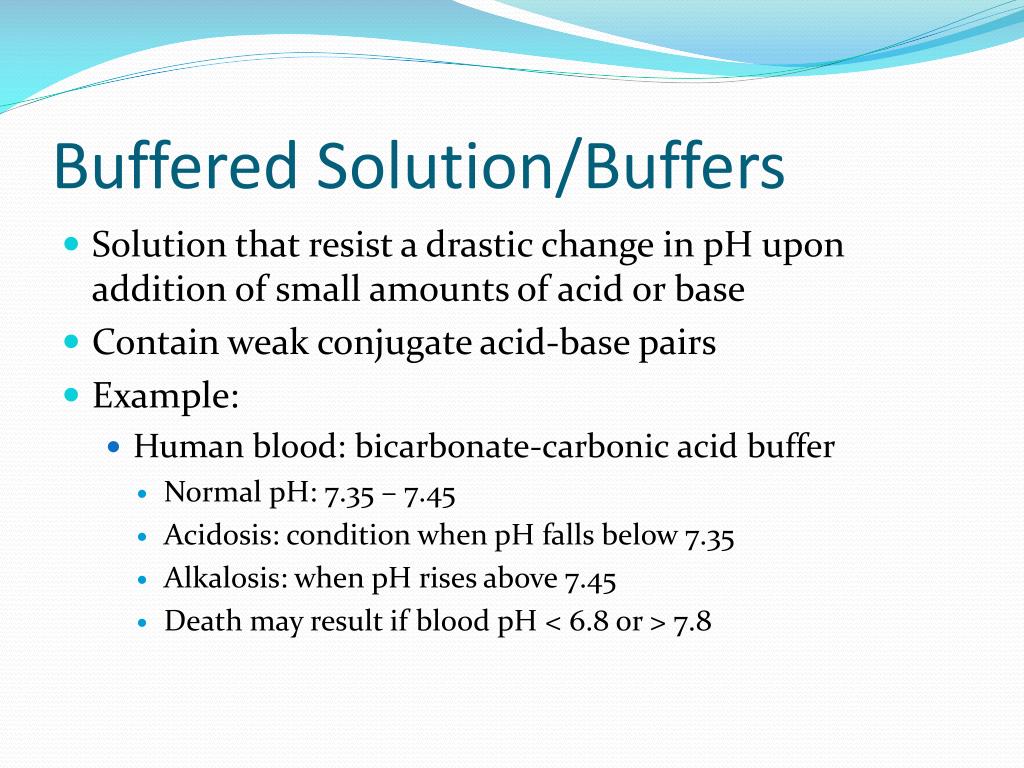
Common Ion Effect In Buffer Solution . To understand how buffers work, let’s look first at how the. This can result in a change in the equilibrium point of the solution. Weak acids combined with conjugate bases. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. How does a buffer work? The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution;
from www.slideserve.com
The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. To understand how buffers work, let’s look first at how the. How does a buffer work? Weak acids combined with conjugate bases. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; This can result in a change in the equilibrium point of the solution.
PPT The CommonIon Effect PowerPoint Presentation, free download ID
Common Ion Effect In Buffer Solution How does a buffer work? Weak acids combined with conjugate bases. This can result in a change in the equilibrium point of the solution. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. To understand how buffers work, let’s look first at how the. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; How does a buffer work?
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Effect In Buffer Solution How does a buffer work? This can result in a change in the equilibrium point of the solution. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic. Common Ion Effect In Buffer Solution.
From www.scribd.com
Common Ion Effect and Buffer Solution PDF Buffer Solution Acid Common Ion Effect In Buffer Solution To understand how buffers work, let’s look first at how the. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. Weak acids combined with conjugate bases. How does a buffer work? The common ion effect is an application of le chatelier's principle to the. Common Ion Effect In Buffer Solution.
From wordmint.com
common ion effect and buffer solution Word Scramble WordMint Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a. Common Ion Effect In Buffer Solution.
From www.youtube.com
Common Ion Effect, Buffers Solutions and Change in pH Part 4 YouTube Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. How does a buffer work? The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Effect In Buffer Solution The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes an ion’s effect on the solubility equilibrium of. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Effect In Buffer Solution How does a buffer work? This can result in a change in the equilibrium point of the solution. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. To understand how buffers work, let’s look first at how the. Weak acids combined with conjugate bases.. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Common Ion Effect and Buffer Solution Presentation Studypool Common Ion Effect In Buffer Solution The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; Weak acids combined with conjugate bases. To understand how buffers work, let’s look first at how the. How does a buffer work? This. Common Ion Effect In Buffer Solution.
From www.youtube.com
COMMON ION EFFECT/BUFFER SOLUTION/BUFFER CAPACITY/SOLUBILITY/SOLUBILITY Common Ion Effect In Buffer Solution The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect is an application of le chatelier's principle to the. Common Ion Effect In Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Effect In Buffer Solution How does a buffer work? If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. The common ion effect describes the effect on equilibrium that occurs when a common ion. Common Ion Effect In Buffer Solution.
From www.studocu.com
Lecture 14 Common ion effect and Buffer Solutions Common ion effect Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. This can. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Ionic equilibrium note 7 buffer solution salt hydrolysis and Common Ion Effect In Buffer Solution The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. To understand how buffers work,. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Analytical chemistry common ion effect buffer solution notes Common Ion Effect In Buffer Solution The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. This can. Common Ion Effect In Buffer Solution.
From www.studocu.com
Buffers notes BUFFER common ion effect The CommonIon Effect Common Ion Effect In Buffer Solution This can result in a change in the equilibrium point of the solution. Weak acids combined with conjugate bases. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If. Common Ion Effect In Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Effect In Buffer Solution This can result in a change in the equilibrium point of the solution. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. To understand how buffers work, let’s look first at how the. How does a buffer work? If a soluble compound consisting of. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Ionic equilibrium note 7 buffer solution salt hydrolysis and Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT The CommonIon Effect PowerPoint Presentation, free download ID Common Ion Effect In Buffer Solution This can result in a change in the equilibrium point of the solution. To understand how buffers work, let’s look first at how the. Weak acids combined with conjugate bases. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. How does a buffer work? If a soluble compound consisting of a common ion is. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Ionic equilibrium note 7 buffer solution salt hydrolysis and Common Ion Effect In Buffer Solution The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. This can result in a change in the equilibrium point of the solution. If a soluble compound consisting of a common ion is added, it can decrease. Common Ion Effect In Buffer Solution.
From slideplayer.com
Common Ion Effect Buffer Solutions The resistance of pH change. ppt Common Ion Effect In Buffer Solution To understand how buffers work, let’s look first at how the. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; This can result in a change in the equilibrium. Common Ion Effect In Buffer Solution.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Common Ion Effect In Buffer Solution This can result in a change in the equilibrium point of the solution. To understand how buffers work, let’s look first at how the. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes the effect on equilibrium that occurs when a common. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Common Ion Effect PowerPoint Presentation, free download ID3469534 Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Effect In Buffer Solution This can result in a change in the equilibrium point of the solution. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. How does a buffer work? The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. To understand. Common Ion Effect In Buffer Solution.
From www.scribd.com
Common Ion Effect and Buffer Solution PDF Buffer Solution Acid Common Ion Effect In Buffer Solution The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes an ion’s effect on the solubility equilibrium of. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Common Ion Effect In Buffer Solution Weak acids combined with conjugate bases. How does a buffer work? This can result in a change in the equilibrium point of the solution. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that. Common Ion Effect In Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Effect In Buffer Solution The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. How does a buffer work? To understand how buffers work, let’s look first at how the. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; Weak acids combined with conjugate bases. The. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Chapter 16 Introduction to Buffers PowerPoint Presentation, free Common Ion Effect In Buffer Solution If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; To understand how buffers work, let’s look first at how the. Weak acids combined with conjugate bases. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. How does a buffer work? The. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Chapter Fourteen PowerPoint Presentation, free download ID4340197 Common Ion Effect In Buffer Solution To understand how buffers work, let’s look first at how the. Weak acids combined with conjugate bases. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. If a soluble compound consisting of a common ion is. Common Ion Effect In Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Effect In Buffer Solution If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; How does a buffer work? The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. This can result in a change in the. Common Ion Effect In Buffer Solution.
From oneclass.com
OneClass 55 of 57 Common ions Effect A buffer solution is 0.24 M NH3 Common Ion Effect In Buffer Solution This can result in a change in the equilibrium point of the solution. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; Weak acids combined with conjugate bases. The common ion effect. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Common Ion Effect and Buffer Solution Presentation Studypool Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. This can result in a change in the equilibrium point of the solution. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect describes the effect on equilibrium that occurs when a common. Common Ion Effect In Buffer Solution.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Common Ion Effect In Buffer Solution The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. How does a buffer work? If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes an ion’s effect on the solubility equilibrium of a substance.. Common Ion Effect In Buffer Solution.
From www.pinterest.com
Common Ion Effect Buffer Solution and Solubility Product Solubility Common Ion Effect In Buffer Solution How does a buffer work? This can result in a change in the equilibrium point of the solution. Weak acids combined with conjugate bases. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. If a soluble compound consisting of a common ion is added,. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 Common Ion Effect In Buffer Solution How does a buffer work? This can result in a change in the equilibrium point of the solution. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect is an application of le chatelier's principle to the equilibrium concentration of ionic compounds. Weak acids. Common Ion Effect In Buffer Solution.
From www.youtube.com
Common Ion Effect and Buffer Solutions YouTube Common Ion Effect In Buffer Solution Weak acids combined with conjugate bases. To understand how buffers work, let’s look first at how the. If a soluble compound consisting of a common ion is added, it can decrease the concentration of that ion within the solution; The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. The common ion effect is an. Common Ion Effect In Buffer Solution.
From www.studypool.com
SOLUTION Ionic equilibrium note 7 buffer solution salt hydrolysis and Common Ion Effect In Buffer Solution How does a buffer work? This can result in a change in the equilibrium point of the solution. The common ion effect describes an ion’s effect on the solubility equilibrium of a substance. To understand how buffers work, let’s look first at how the. Weak acids combined with conjugate bases. The common ion effect describes the effect on equilibrium that. Common Ion Effect In Buffer Solution.
From www.slideserve.com
PPT Ionic Equilibria 離子平衡 Part II Buffers 緩衝液 and Titration Curves Common Ion Effect In Buffer Solution How does a buffer work? The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the solution) is. Weak acids combined with conjugate bases. To understand how buffers work, let’s look first at how the. This can result in a change in the equilibrium point of the solution.. Common Ion Effect In Buffer Solution.