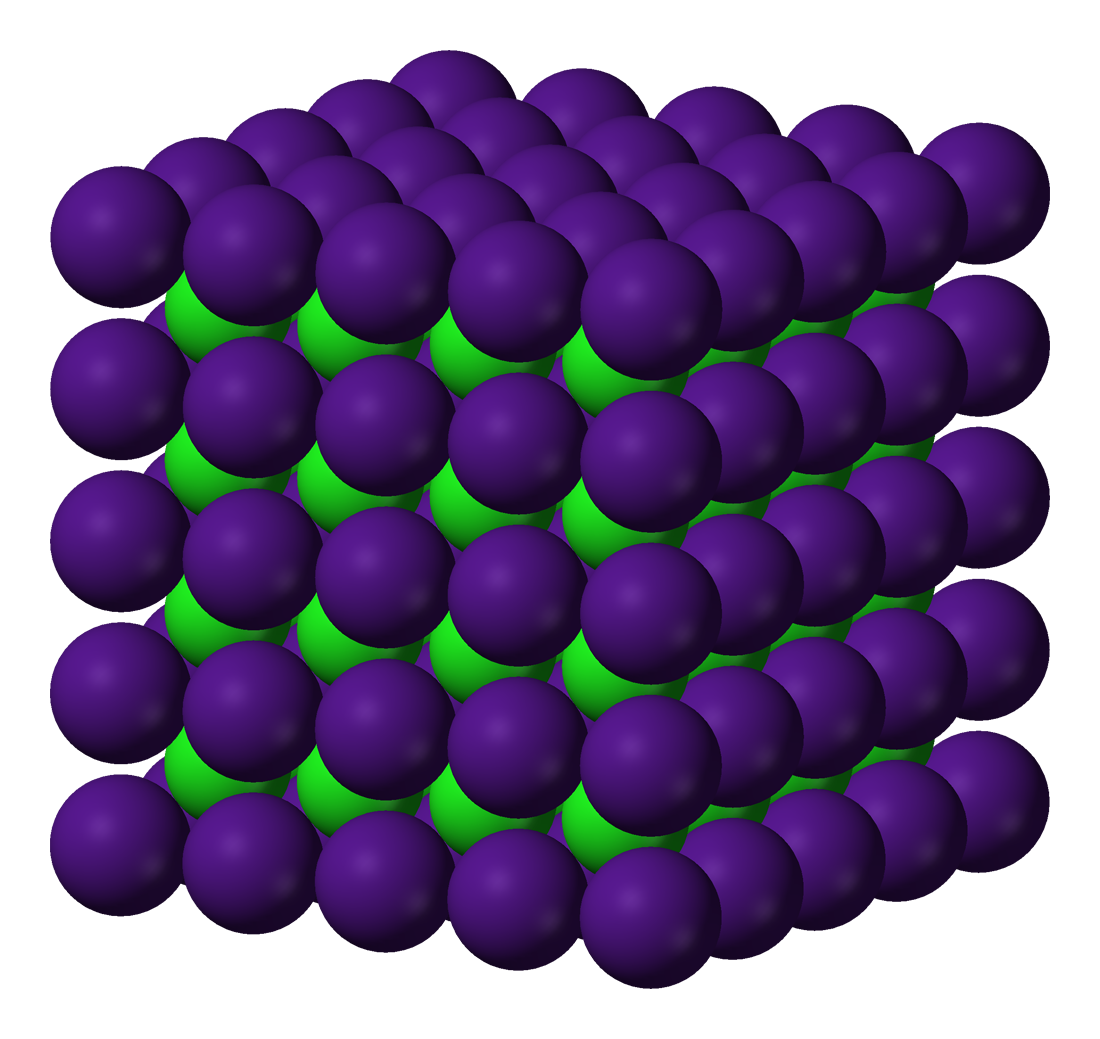
Crystals Metallic Examples . Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. A classic example is a halite or salt crystal. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. A single metal can form multiple types of metallic crystals. These metallic bonds are what give metals. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Iron, for example, can form different metallic crystals. Electrostatic forces form ionic bonds. Like ionic solids, metals and alloys have a very strong. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metallic crystals are made of metals and held together using metallic bonds. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to.
from chem.libretexts.org
A single metal can form multiple types of metallic crystals. Like ionic solids, metals and alloys have a very strong. Electrostatic forces form ionic bonds. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Iron, for example, can form different metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. These metallic bonds are what give metals. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. A classic example is a halite or salt crystal.
Crystalline Solid Structures Chemistry LibreTexts
Crystals Metallic Examples These metallic bonds are what give metals. Iron, for example, can form different metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. Electrostatic forces form ionic bonds. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Metallic crystals are made of metals and held together using metallic bonds. These metallic bonds are what give metals. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. A single metal can form multiple types of metallic crystals. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Like ionic solids, metals and alloys have a very strong. A classic example is a halite or salt crystal.
From celestialearthminerals.com
Quartz Crystal Mineral Specimen Celestial Earth Minerals Crystals Metallic Examples Like ionic solids, metals and alloys have a very strong. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Electrostatic forces form ionic bonds. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. These crystals have a shiny appearance and include copper, gold, aluminum, and iron,. Crystals Metallic Examples.
From saylordotorg.github.io
Crystalline and Amorphous Solids Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. A single metal can form multiple types of metallic crystals. These metallic bonds are what give metals. All metallic elements (except cs, ga, and hg) are crystalline solids. Crystals Metallic Examples.
From www.schoolchalao.com
Metallic Minerals Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A single metal can form multiple types of metallic crystals. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which. Crystals Metallic Examples.
From www.pinterest.com.au
Crystals of Platinum metal Minerals and gemstones, Gems and minerals Crystals Metallic Examples These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Like ionic solids, metals and alloys have a very strong. A single metal can form multiple types of metallic crystals. Iron, for example, can form different metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice.. Crystals Metallic Examples.
From thatcrystalsite.com
15 Of The Best Metallic Crystals That Crystal Site Crystals Metallic Examples Iron, for example, can form different metallic crystals. Electrostatic forces form ionic bonds. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Metallic crystals are made of metals and held together using metallic bonds. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. A single metal can. Crystals Metallic Examples.
From www.greenqueen.com.hk
6 Things To Know Before You Buy A Healing Crystal Crystals Metallic Examples A classic example is a halite or salt crystal. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A single metal can form multiple types of metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Electrostatic forces form ionic bonds.. Crystals Metallic Examples.
From www.pinterest.com
Download Raw Gemstone Identification Chart Rock minerals, Rocks and Crystals Metallic Examples A single metal can form multiple types of metallic crystals. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. A classic example is a halite or salt crystal. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Iron, for example, can form different metallic crystals. Like. Crystals Metallic Examples.
From news.mit.edu
Turning diamond into metal MIT News Massachusetts Institute of Crystals Metallic Examples These metallic bonds are what give metals. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. A classic example is a halite or salt crystal. Metallic crystals are made of metals and held together using metallic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the. Crystals Metallic Examples.
From www.thoughtco.com
10 Minerals That Have Metallic Luster Crystals Metallic Examples Iron, for example, can form different metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Like ionic solids, metals and alloys have a very strong. A classic example is a halite or salt crystal. Metals often form metallic crystals, where some of the valence electrons are free to move. Crystals Metallic Examples.
From celestialearthminerals.com
Quartz Crystal Mineral Specimen Celestial Earth Minerals Crystals Metallic Examples These metallic bonds are what give metals. A classic example is a halite or salt crystal. Iron, for example, can form different metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice.. Crystals Metallic Examples.
From mallcrawlersinc.com
Granite Crystal Metallic JL Mall Crawlers Inc Crystals Metallic Examples These metallic bonds are what give metals. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Metallic crystals are made of metals and held together using. Crystals Metallic Examples.
From www.slideserve.com
PPT Structures of Simple Solids PowerPoint Presentation, free Crystals Metallic Examples Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. Iron, for example, can form different metallic crystals. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. A single metal can form multiple types of metallic crystals. These metallic bonds are what give metals. Like ionic solids, metals. Crystals Metallic Examples.
From www.youtube.com
Metallic Crystals The Solid State CBSE Class 12 Chemistry YouTube Crystals Metallic Examples These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. A single metal can form multiple types of metallic crystals. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Electrostatic forces form ionic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the. Crystals Metallic Examples.
From elite-dangerous.fandom.com
Metallic Crystal Elite Dangerous Wiki Fandom Crystals Metallic Examples Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Iron, for example, can form different metallic crystals. A classic example is a halite or salt crystal. All metallic elements (except cs, ga, and hg) are crystalline. Crystals Metallic Examples.
From www.pinterest.ph
Pirite also known as fool's gold, this mineral's metallic luster and Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A classic example is a halite or salt crystal. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds.. Crystals Metallic Examples.
From mallcrawlersinc.com
Granite Crystal Metallic JL Mall Crawlers Inc Crystals Metallic Examples Metallic crystals are made of metals and held together using metallic bonds. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. A classic example is a halite or salt crystal. Like ionic solids, metals and alloys have a very strong. Metallic crystals are also atomic solids, made of metal atoms held together. Crystals Metallic Examples.
From www.thoughtco.com
Crystal Definition, Examples, and Common Types Crystals Metallic Examples Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. A single metal can form multiple types of metallic crystals. These metallic bonds are what give metals.. Crystals Metallic Examples.
From www.vitabeads.com
Metallic Crystal Crystals Metallic Examples A single metal can form multiple types of metallic crystals. Electrostatic forces form ionic bonds. Iron, for example, can form different metallic crystals. Metallic crystals are made of metals and held together using metallic bonds. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Like ionic solids, metals and alloys have a very strong.. Crystals Metallic Examples.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Crystals Metallic Examples Electrostatic forces form ionic bonds. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metals often form metallic crystals, where some of. Crystals Metallic Examples.
From www.irocks.com
Splendent, Silvery Arsenopyrite Crystal Mass iRocks Fine Minerals Crystals Metallic Examples These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. A single metal can form multiple types of metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Like ionic solids, metals. Crystals Metallic Examples.
From www.fossilera.com
4.1" Metallic Stibnite Crystal Cluster China (93679) For Sale Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metallic crystals are made of metals and held together using metallic bonds. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Electrostatic forces form ionic bonds. Like ionic solids, metals and alloys have a very strong. Metals. Crystals Metallic Examples.
From crystal-information.com
Copper Properties and Meaning + Photos Crystal Information Crystals Metallic Examples All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Metallic crystals are made of metals and held together using metallic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A single metal can form multiple types of metallic crystals. Like ionic solids, metals and alloys. Crystals Metallic Examples.
From www.thoughtco.com
Metal Crystals Photo Gallery Crystals Metallic Examples Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. Electrostatic forces form ionic bonds. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Like ionic solids, metals and alloys have a very. Crystals Metallic Examples.
From www.pinterest.com
Pure Silver Dendritic Crystal Crystals, Gems and minerals, Silver Crystals Metallic Examples These metallic bonds are what give metals. Like ionic solids, metals and alloys have a very strong. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Iron, for example, can form different metallic crystals. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. All metallic elements. Crystals Metallic Examples.
From www.thoughtco.com
Metal Crystals Photo Gallery Crystals Metallic Examples Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. These metallic bonds are what give metals. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Iron, for example, can form different metallic crystals. Electrostatic forces form ionic bonds. Like ionic solids, metals and alloys have a very strong. Metallic. Crystals Metallic Examples.
From www.thoughtco.com
Metal Crystals Photo Gallery Crystals Metallic Examples Like ionic solids, metals and alloys have a very strong. Iron, for example, can form different metallic crystals. Metallic crystals are made of metals and held together using metallic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A classic example is a halite or salt crystal. These metallic bonds. Crystals Metallic Examples.
From rhinestones.com
Crystal Metallic Light Gold F 14x10mm Crystal Metallic Light Gold F Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Electrostatic forces form ionic bonds. Metals often form metallic crystals,. Crystals Metallic Examples.
From vitabeads.com
Metallic Crystal Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Like ionic solids, metals and alloys have a very strong.. Crystals Metallic Examples.
From slideplayer.com
Solids. ppt download Crystals Metallic Examples These metallic bonds are what give metals. These crystals have a shiny appearance and include copper, gold, aluminum, and iron, to. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A single metal can form multiple types of metallic crystals. Electrostatic forces form ionic bonds. Metals often form metallic crystals, where. Crystals Metallic Examples.
From www.youtube.com
These Hybrid Metallic Crystals Are Chemistry’s New Miracle Materials Crystals Metallic Examples Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Electrostatic forces form ionic bonds. Like ionic solids, metals and alloys have a very strong. A single metal can form multiple types of metallic crystals. All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. These crystals have. Crystals Metallic Examples.
From chemistry.about.com
Types of Crystals Shapes and Structures Crystals Metallic Examples Electrostatic forces form ionic bonds. Iron, for example, can form different metallic crystals. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. These metallic bonds are what give metals. A classic example is a halite or salt crystal. A single metal can form multiple types of metallic crystals. Metals often form. Crystals Metallic Examples.
From www.quiltersexpress.com
Crystal Metallic Sunshine 001METSH 16ss/4mm Swarovski Flat Back Crystals Metallic Examples Metallic crystals are formed by metal atoms sharing a “sea” of delocalized electrons, which allows them to. A single metal can form multiple types of metallic crystals. Metallic crystals are made of metals and held together using metallic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A classic example. Crystals Metallic Examples.
From www.thoughtco.com
Metal Crystals Photo Gallery Crystals Metallic Examples A classic example is a halite or salt crystal. Metallic crystals are also atomic solids, made of metal atoms held together by metallic bonds. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Electrostatic forces form ionic bonds. Like ionic solids, metals and alloys have a very strong. These metallic bonds. Crystals Metallic Examples.
From www.pinterest.com
Examples of Different Mineral Lusters Pyrite, Fool gold, Real gold Crystals Metallic Examples All metallic elements (except cs, ga, and hg) are crystalline solids at room temperature. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. Iron, for example, can form different metallic crystals. Metallic crystals. Crystals Metallic Examples.
From www.crystalandglassbeads.com
Crystal Metallic Blue ss12 Swarovski Flatback Crystals 2088 Non Hotfix Crystals Metallic Examples Iron, for example, can form different metallic crystals. These metallic bonds are what give metals. Like ionic solids, metals and alloys have a very strong. Metals often form metallic crystals, where some of the valence electrons are free to move throughout the lattice. A classic example is a halite or salt crystal. Metallic crystals are made of metals and held. Crystals Metallic Examples.