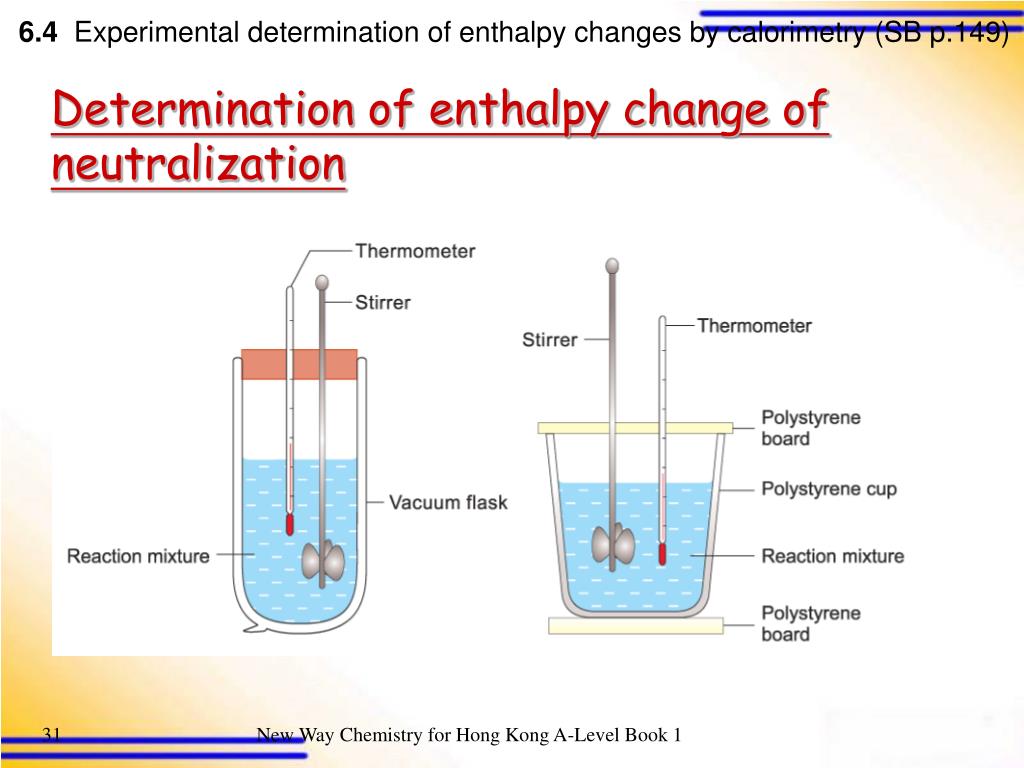
Experiment Calorimetry And Heat Of Neutralization . The enthalpy change of acid base neutralization reactions will be determined. This is an iot lab, where you will be asked to. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. This experiment introduces the technique of calorimetry. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). We used a constructed calorimeter and.
from www.slideserve.com
Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The enthalpy change of acid base neutralization reactions will be determined. We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). This is an iot lab, where you will be asked to. This experiment introduces the technique of calorimetry. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings).
PPT Energetics PowerPoint Presentation, free download ID1204656
Experiment Calorimetry And Heat Of Neutralization The enthalpy change of acid base neutralization reactions will be determined. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The enthalpy change of acid base neutralization reactions will be determined. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. We used a constructed calorimeter and. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). This experiment introduces the technique of calorimetry. This is an iot lab, where you will be asked to. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings).
From www.studocu.com
Experiment 10(Heat of Neutralization) SubjectPhysical Chemistry Lab Experiment Calorimetry And Heat Of Neutralization This is an iot lab, where you will be asked to. The enthalpy change of acid base neutralization reactions will be determined. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained. Experiment Calorimetry And Heat Of Neutralization.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Experiment Calorimetry And Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction proceeds in an adiabatic. Experiment Calorimetry And Heat Of Neutralization.
From www.youtube.com
2.1 Calorimetry Heat of neutralization YouTube Experiment Calorimetry And Heat Of Neutralization This experiment introduces the technique of calorimetry. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel.. Experiment Calorimetry And Heat Of Neutralization.
From www.pdffiller.com
Fillable Online EXPERIMENT CALORIMETRY AND HEAT OF NEUTRALIZATION Experiment Calorimetry And Heat Of Neutralization We used a constructed calorimeter and. This is an iot lab, where you will be asked to. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by. Experiment Calorimetry And Heat Of Neutralization.
From www.scribd.com
Experiment 6 Calorimetry Determination of Heats of Neutralization Experiment Calorimetry And Heat Of Neutralization If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. This experiment introduces the technique of calorimetry. This is an iot lab, where. Experiment Calorimetry And Heat Of Neutralization.
From www.studocu.com
Calorimetry anf heat of neutralization exp BSc V sem Revised 11/2015 Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). This experiment introduces the technique of calorimetry. If. Experiment Calorimetry And Heat Of Neutralization.
From www.youtube.com
Neutralization Calorimetry Examples YouTube Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The enthalpy change of acid base neutralization reactions will be determined. We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its. Experiment Calorimetry And Heat Of Neutralization.
From studylib.net
Calorimetry Heat of Neutralisation Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is lost in the chemical reaction (the system). Experiment Calorimetry And Heat Of Neutralization.
From www.learnsci.com
LearnSci LabSim Calorimeter for Measuring Enthalpy Change of Experiment Calorimetry And Heat Of Neutralization The enthalpy change of acid base neutralization reactions will be determined. We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is. Experiment Calorimetry And Heat Of Neutralization.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Experiment Calorimetry And Heat Of Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The enthalpy change of acid base neutralization reactions will be determined. Introduction to the. Experiment Calorimetry And Heat Of Neutralization.
From www.scribd.com
Determination of Heat of Neutralization Using Calorimetry and Analysis Experiment Calorimetry And Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is. Experiment Calorimetry And Heat Of Neutralization.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Experiment Calorimetry And Heat Of Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The enthalpy change of acid base neutralization reactions will be determined. If a reaction. Experiment Calorimetry And Heat Of Neutralization.
From studylib.net
Calorimetry Heat of Neutralization Experiment Calorimetry And Heat Of Neutralization This is an iot lab, where you will be asked to. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). We used. Experiment Calorimetry And Heat Of Neutralization.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Experiment Calorimetry And Heat Of Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The enthalpy change of acid base neutralization reactions will be determined. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction. Experiment Calorimetry And Heat Of Neutralization.
From guweb2.gonzaga.edu
CHEM 101 General Chemistry topic Experiment Calorimetry And Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction proceeds in an adiabatic. Experiment Calorimetry And Heat Of Neutralization.
From www.scribd.com
Heat of Neutralization Lab Experiment Calorimetry And Heat Of Neutralization If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The heat of neutralization that is lost in the chemical reaction (the system). Experiment Calorimetry And Heat Of Neutralization.
From www.youtube.com
Neutralization Calorimetry Experiment YouTube Experiment Calorimetry And Heat Of Neutralization This experiment introduces the technique of calorimetry. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The enthalpy change of acid base neutralization reactions will be determined. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs. Experiment Calorimetry And Heat Of Neutralization.
From chem2u.blogspot.com
chem2U Heat of Neutralisation Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. We used a constructed calorimeter and. Introduction to the technique of calorimetry, in which. Experiment Calorimetry And Heat Of Neutralization.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Experiment Calorimetry And Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. The enthalpy change of acid base neutralization reactions will be determined. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and. Experiment Calorimetry And Heat Of Neutralization.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. This experiment introduces the technique of calorimetry.. Experiment Calorimetry And Heat Of Neutralization.
From www.chegg.com
Solved EXPERIMENT REPORT SHEET Heat of Neutralization A. Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. We used a constructed calorimeter and. Introduction to the technique of calorimetry, in which. Experiment Calorimetry And Heat Of Neutralization.
From www.researchgate.net
(PDF) “Greening” a Familiar General Chemistry Experiment Coffee Cup Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. The heat of neutralization that is lost. Experiment Calorimetry And Heat Of Neutralization.
From vdocuments.site
EXPERIMENT CALORIMETRY AND HEAT OF NEUTRALIZATIONfaculty.cbu.ca Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is lost in the chemical reaction (the system). Experiment Calorimetry And Heat Of Neutralization.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Experiment Calorimetry And Heat Of Neutralization This experiment introduces the technique of calorimetry. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. The enthalpy change of acid base neutralization reactions will be determined. If a reaction proceeds in an adiabatic calorimeter, we can calculate the. Experiment Calorimetry And Heat Of Neutralization.
From www.youtube.com
Heat of Neutralization Part 1 Thermochemistry YouTube Experiment Calorimetry And Heat Of Neutralization We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). This is an iot lab, where you will be asked to. This experiment introduces the technique of calorimetry. The heat of neutralization that is lost in the chemical reaction (the system). Experiment Calorimetry And Heat Of Neutralization.
From www.transtutors.com
(Solved) REPORT SHEET EXPERIMENT 28 Heat Of Neutralization °C A. Heat Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). We used a constructed calorimeter and. The enthalpy change of acid base neutralization reactions will be determined. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its. Experiment Calorimetry And Heat Of Neutralization.
From www.studypool.com
SOLUTION Calorimetry lattice energy heat of neutralization atomisation Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. This is an iot lab, where you will be asked to. The enthalpy change. Experiment Calorimetry And Heat Of Neutralization.
From www.slideserve.com
PPT Heat of Neutralization PowerPoint Presentation, free download Experiment Calorimetry And Heat Of Neutralization We used a constructed calorimeter and. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The enthalpy change of acid base neutralization reactions. Experiment Calorimetry And Heat Of Neutralization.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or. Experiment Calorimetry And Heat Of Neutralization.
From www.numerade.com
SOLVEDREPORT SHEET Heat of Neutralization EXPERIMENT 12 Heat Capacity Experiment Calorimetry And Heat Of Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). This is an iot lab, where you will be asked to. This experiment introduces the technique of calorimetry. We used a constructed calorimeter and. Abstract the purpose of this experiment was to determine the heat capacity of. Experiment Calorimetry And Heat Of Neutralization.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Experiment Calorimetry And Heat Of Neutralization The enthalpy change of acid base neutralization reactions will be determined. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat. Experiment Calorimetry And Heat Of Neutralization.
From www.studypool.com
SOLUTION Heat capacity of calorimeter and enthalpy of neutralization Experiment Calorimetry And Heat Of Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The enthalpy change of acid base neutralization reactions will be determined. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in. Experiment Calorimetry And Heat Of Neutralization.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID1204656 Experiment Calorimetry And Heat Of Neutralization We used a constructed calorimeter and. This is an iot lab, where you will be asked to. If a reaction proceeds in an adiabatic calorimeter, we can calculate the heat that the reaction releases or absorbs by measuring the change in. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and. Experiment Calorimetry And Heat Of Neutralization.
From www.chegg.com
Solved EXPERIMENT REPORT SHEET Heat of Neutralization A. Experiment Calorimetry And Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The heat of neutralization that is lost. Experiment Calorimetry And Heat Of Neutralization.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Experiment Calorimetry And Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. This is an iot lab, where you. Experiment Calorimetry And Heat Of Neutralization.