Why Does Salt Melt Ice Fastest . Because salt particles make it harder for water particles to freeze back onto the ice, the. Salt makes ice colder because the salt prevents melted water from freezing. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Salt lowers the freezing point of water. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. Let’s start with salt’s relationship. When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. This behavior of water makes it possible to use salt to melt ice. Why does salt melt ice? The salt has to dissolve into its ions in order to work. But there’s plenty more to it than that, so we consulted the experts.
from whatmakesicemeltfastest.weebly.com
Because salt particles make it harder for water particles to freeze back onto the ice, the. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression. Salt lowers the freezing point of water. But there’s plenty more to it than that, so we consulted the experts. The salt has to dissolve into its ions in order to work. Let’s start with salt’s relationship. Salt only helps if there is a little bit of liquid water available. Melting is endothermic, so it lowers the temperature. Why does salt melt ice?
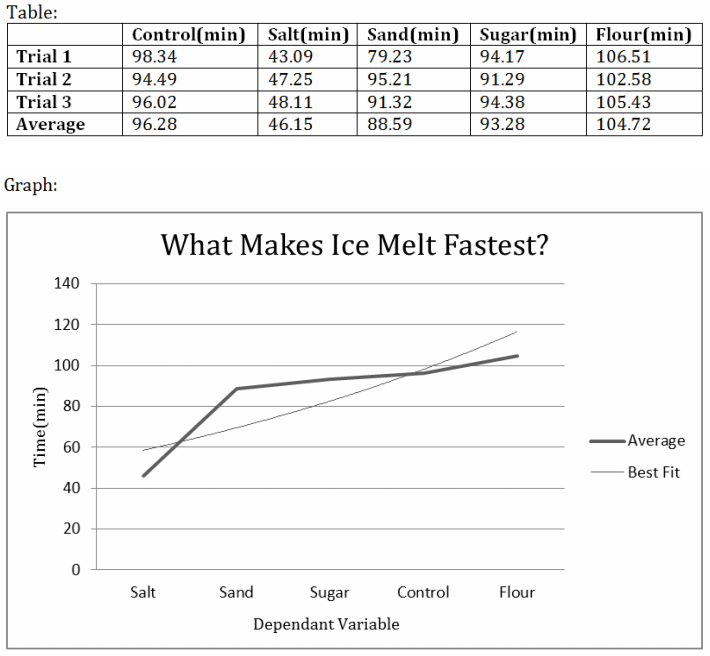
Formal Experimental Record WHat makes ice melt fastest???
Why Does Salt Melt Ice Fastest Because salt particles make it harder for water particles to freeze back onto the ice, the. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Why does salt melt ice? When ice melts, water and ice coexist. Salt helps melt ice and prevent it. But there’s plenty more to it than that, so we consulted the experts. Salt lowers the freezing point of water. This phenomenon is called freezing point depression. Let’s start with salt’s relationship. The salt has to dissolve into its ions in order to work. Salt makes ice colder because the salt prevents melted water from freezing. This behavior of water makes it possible to use salt to melt ice. Because salt particles make it harder for water particles to freeze back onto the ice, the. Salt only helps if there is a little bit of liquid water available. Melting is endothermic, so it lowers the temperature.
From giokhlnfc.blob.core.windows.net
Why Does Snow Melt On Roads at Henry Culp blog Why Does Salt Melt Ice Fastest For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. This behavior of water makes it possible to use salt to melt ice. Melting is endothermic, so it lowers the temperature. But there’s plenty more to it than that, so we consulted the experts. Salt. Why Does Salt Melt Ice Fastest.
From magicgouveiapetters.z21.web.core.windows.net
Why Does Adding Salt To Ice Make It Melt Why Does Salt Melt Ice Fastest The salt has to dissolve into its ions in order to work. Melting is endothermic, so it lowers the temperature. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Salt helps melt ice and prevent it. This behavior of water makes it possible to. Why Does Salt Melt Ice Fastest.
From www.wearegreenbay.com
What melts ice the fastest? Science Course with Storm Team 5 WFRV Why Does Salt Melt Ice Fastest Melting is endothermic, so it lowers the temperature. But there’s plenty more to it than that, so we consulted the experts. Why does salt melt ice? Salt lowers the freezing point of water. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. This behavior. Why Does Salt Melt Ice Fastest.
From musicbykatie.com
Does Salt Make Ice Melt The Fastest? Best 28 Answer Why Does Salt Melt Ice Fastest Salt only helps if there is a little bit of liquid water available. Why does salt melt ice? Let’s start with salt’s relationship. Melting is endothermic, so it lowers the temperature. Salt makes ice colder because the salt prevents melted water from freezing. Salt helps melt ice and prevent it. When ice melts, water and ice coexist. For the salt. Why Does Salt Melt Ice Fastest.
From www.science-sparks.com
Why does salt melt ice? Why Does Salt Melt Ice Fastest Salt makes ice colder because the salt prevents melted water from freezing. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Salt helps melt ice and prevent it. The salt has to dissolve into its ions in order to work. When ice melts, water. Why Does Salt Melt Ice Fastest.
From materialmcgheepearter.z21.web.core.windows.net
Why Does Ice Melt With Salt Why Does Salt Melt Ice Fastest Salt lowers the freezing point of water. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. When ice melts, water and ice coexist. But there’s plenty more to it than that, so we consulted the experts. Why. Why Does Salt Melt Ice Fastest.
From www.sciencekiddo.com
Why Salt Melts Ice Easy Science for Kids Science Kiddo Why Does Salt Melt Ice Fastest But there’s plenty more to it than that, so we consulted the experts. Salt lowers the freezing point of water. Salt makes ice colder because the salt prevents melted water from freezing. Salt helps melt ice and prevent it. Salt only helps if there is a little bit of liquid water available. Let’s start with salt’s relationship. Melting is endothermic,. Why Does Salt Melt Ice Fastest.
From www.pinterest.com
What Makes Ice Melt Fastest? Science projects, Science fair, Science Why Does Salt Melt Ice Fastest Because salt particles make it harder for water particles to freeze back onto the ice, the. When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. But there’s plenty more to it than that, so we consulted the experts. The salt has to dissolve into its ions in. Why Does Salt Melt Ice Fastest.
From chemistry.about.com
Why Does Salt Melt Ice? Understanding How It Works Why Does Salt Melt Ice Fastest But there’s plenty more to it than that, so we consulted the experts. Salt helps melt ice and prevent it. This behavior of water makes it possible to use salt to melt ice. Let’s start with salt’s relationship. Why does salt melt ice? Salt makes ice colder because the salt prevents melted water from freezing. For the salt spread on. Why Does Salt Melt Ice Fastest.
From dozorisozo.github.io
How To Draw Melting Ice Cube Ice Science Melts Fastest Fair Melt Why Does Salt Melt Ice Fastest This behavior of water makes it possible to use salt to melt ice. Salt lowers the freezing point of water. Salt helps melt ice and prevent it. Because salt particles make it harder for water particles to freeze back onto the ice, the. For the salt spread on streets, lowering the freezing point means that ice can melt even when. Why Does Salt Melt Ice Fastest.
From stanley-kbryant.blogspot.com
Why Does Salt Melt Ice Faster Than Sand Why Does Salt Melt Ice Fastest Because salt particles make it harder for water particles to freeze back onto the ice, the. Melting is endothermic, so it lowers the temperature. Salt lowers the freezing point of water. Salt makes ice colder because the salt prevents melted water from freezing. Let’s start with salt’s relationship. Why does salt melt ice? The salt has to dissolve into its. Why Does Salt Melt Ice Fastest.
From whatmakesicemeltfastest.weebly.com
Formal Experimental Record WHat makes ice melt fastest??? Why Does Salt Melt Ice Fastest When ice melts, water and ice coexist. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Why does salt melt ice? Let’s start with salt’s relationship. But there’s plenty more to it than that, so we consulted the experts. Salt only helps if there. Why Does Salt Melt Ice Fastest.
From giothvwfr.blob.core.windows.net
How To Use Salt To Melt Ice at Emmett Hansen blog Why Does Salt Melt Ice Fastest When ice melts, water and ice coexist. Because salt particles make it harder for water particles to freeze back onto the ice, the. Let’s start with salt’s relationship. This behavior of water makes it possible to use salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression.. Why Does Salt Melt Ice Fastest.
From lessonliblandaulets.z21.web.core.windows.net
Why Does Salt Melt Ice Chemistry Why Does Salt Melt Ice Fastest This phenomenon is called freezing point depression. Let’s start with salt’s relationship. Melting is endothermic, so it lowers the temperature. Salt helps melt ice and prevent it. When ice melts, water and ice coexist. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Why. Why Does Salt Melt Ice Fastest.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Why Does Salt Melt Ice Fastest Why does salt melt ice? Salt lowers the freezing point of water. Salt only helps if there is a little bit of liquid water available. Salt makes ice colder because the salt prevents melted water from freezing. Salt helps melt ice and prevent it. Let’s start with salt’s relationship. For the salt spread on streets, lowering the freezing point means. Why Does Salt Melt Ice Fastest.
From giobwfuik.blob.core.windows.net
Does Salt Dissolve Ice Faster at Charlie Marriott blog Why Does Salt Melt Ice Fastest Salt lowers the freezing point of water. Salt only helps if there is a little bit of liquid water available. Why does salt melt ice? This phenomenon is called freezing point depression. Melting is endothermic, so it lowers the temperature. Salt makes ice colder because the salt prevents melted water from freezing. This behavior of water makes it possible to. Why Does Salt Melt Ice Fastest.
From nateandrachael.com
Baby, It’s Cold Outside! Simple and Playful Ice Experiments Nothing Why Does Salt Melt Ice Fastest But there’s plenty more to it than that, so we consulted the experts. Salt makes ice colder because the salt prevents melted water from freezing. Salt only helps if there is a little bit of liquid water available. Melting is endothermic, so it lowers the temperature. When ice melts, water and ice coexist. Salt helps melt ice and prevent it.. Why Does Salt Melt Ice Fastest.
From frugalfun4boys.com
Melting Ice Science Experiments {Fun!} Frugal Fun For Boys and Girls Why Does Salt Melt Ice Fastest This behavior of water makes it possible to use salt to melt ice. Salt lowers the freezing point of water. Because salt particles make it harder for water particles to freeze back onto the ice, the. Melting is endothermic, so it lowers the temperature. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called. Why Does Salt Melt Ice Fastest.
From www.youtube.com
which ice cube melts fastest? YouTube Why Does Salt Melt Ice Fastest This phenomenon is called freezing point depression. When ice melts, water and ice coexist. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. This behavior of water makes it possible to use salt to melt ice. But there’s plenty more to it than that,. Why Does Salt Melt Ice Fastest.
From fox17.com
Behind the Science Why Salt is Used to Melt Ice WZTV Why Does Salt Melt Ice Fastest Salt lowers the freezing point of water. Why does salt melt ice? Let’s start with salt’s relationship. Salt helps melt ice and prevent it. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. This behavior of water makes it possible to use salt to melt ice. For the salt spread. Why Does Salt Melt Ice Fastest.
From giorraqvn.blob.core.windows.net
Does Clear Ice Melt Slower at Mary Michel blog Why Does Salt Melt Ice Fastest Salt only helps if there is a little bit of liquid water available. Why does salt melt ice? When ice melts, water and ice coexist. This phenomenon is called freezing point depression. This behavior of water makes it possible to use salt to melt ice. Melting is endothermic, so it lowers the temperature. For the salt spread on streets, lowering. Why Does Salt Melt Ice Fastest.
From stanley-kbryant.blogspot.com
Why Does Salt Melt Ice Faster Than Sand Why Does Salt Melt Ice Fastest This phenomenon is called freezing point depression. Salt lowers the freezing point of water. The salt has to dissolve into its ions in order to work. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. This behavior of water makes it possible to use. Why Does Salt Melt Ice Fastest.
From www.youtube.com
How Does Salt Melt Ice? YouTube Why Does Salt Melt Ice Fastest This behavior of water makes it possible to use salt to melt ice. Melting is endothermic, so it lowers the temperature. Salt lowers the freezing point of water. Salt makes ice colder because the salt prevents melted water from freezing. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature. Why Does Salt Melt Ice Fastest.
From exokjpmgr.blob.core.windows.net
Will Salt Melt Ice at Richard Weaver blog Why Does Salt Melt Ice Fastest Salt lowers the freezing point of water. Salt only helps if there is a little bit of liquid water available. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. Why does salt melt ice? Let’s start with salt’s relationship. Melting is endothermic, so it. Why Does Salt Melt Ice Fastest.
From www.wearegreenbay.com
What melts ice the fastest? Science Course with Ryan Morse Why Does Salt Melt Ice Fastest When ice melts, water and ice coexist. This behavior of water makes it possible to use salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression. Why does salt melt ice? Salt lowers the freezing point of water. But there’s plenty more to it than that, so. Why Does Salt Melt Ice Fastest.
From giokhlnfc.blob.core.windows.net
Why Does Snow Melt On Roads at Henry Culp blog Why Does Salt Melt Ice Fastest Salt helps melt ice and prevent it. This behavior of water makes it possible to use salt to melt ice. Let’s start with salt’s relationship. Because salt particles make it harder for water particles to freeze back onto the ice, the. Melting is endothermic, so it lowers the temperature. Salt only helps if there is a little bit of liquid. Why Does Salt Melt Ice Fastest.
From stanley-kbryant.blogspot.com
Why Does Salt Melt Ice Faster Than Sand Why Does Salt Melt Ice Fastest Let’s start with salt’s relationship. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. Because salt particles make it harder for water particles to freeze back onto the ice, the. The salt has to dissolve into its ions in order to work. This behavior of water makes it possible. Why Does Salt Melt Ice Fastest.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED Why Does Salt Melt Ice Fastest Let’s start with salt’s relationship. Salt helps melt ice and prevent it. Because salt particles make it harder for water particles to freeze back onto the ice, the. When ice melts, water and ice coexist. Salt lowers the freezing point of water. Why does salt melt ice? For the salt spread on streets, lowering the freezing point means that ice. Why Does Salt Melt Ice Fastest.
From exoqspafy.blob.core.windows.net
Does Vinegar Melt Ice Faster Than Salt at Deborah Jay blog Why Does Salt Melt Ice Fastest Salt lowers the freezing point of water. Let’s start with salt’s relationship. The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. Why does salt melt ice? When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. For the salt. Why Does Salt Melt Ice Fastest.
From doesitsnowin.com
Why Does Salt Melt Ice? Everything You Need To Know Why Does Salt Melt Ice Fastest Salt makes ice colder because the salt prevents melted water from freezing. The salt has to dissolve into its ions in order to work. For the salt spread on streets, lowering the freezing point means that ice can melt even when the outdoor temperature is below water’s freezing point. This phenomenon is called freezing point depression. Salt lowers the freezing. Why Does Salt Melt Ice Fastest.
From www.youtube.com
Why Does Salt Melt Ice? SCI CODE YouTube Why Does Salt Melt Ice Fastest Let’s start with salt’s relationship. Salt makes ice colder because the salt prevents melted water from freezing. Because salt particles make it harder for water particles to freeze back onto the ice, the. Salt only helps if there is a little bit of liquid water available. Salt lowers the freezing point of water. But there’s plenty more to it than. Why Does Salt Melt Ice Fastest.
From reesesicecreamdobunbu.blogspot.com
Reese S Ice Cream What Melts Ice The Fastest Salt Or Sugar Why Does Salt Melt Ice Fastest This phenomenon is called freezing point depression. When ice melts, water and ice coexist. Let’s start with salt’s relationship. Because salt particles make it harder for water particles to freeze back onto the ice, the. Why does salt melt ice? Salt helps melt ice and prevent it. Salt lowers the freezing point of water. Salt makes ice colder because the. Why Does Salt Melt Ice Fastest.
From www.sciencebuddies.org
What Makes Ice Melt Fastest? Why Does Salt Melt Ice Fastest Let’s start with salt’s relationship. But there’s plenty more to it than that, so we consulted the experts. When ice melts, water and ice coexist. Melting is endothermic, so it lowers the temperature. This behavior of water makes it possible to use salt to melt ice. Why does salt melt ice? For the salt spread on streets, lowering the freezing. Why Does Salt Melt Ice Fastest.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden Why Does Salt Melt Ice Fastest This phenomenon is called freezing point depression. Why does salt melt ice? Let’s start with salt’s relationship. Salt helps melt ice and prevent it. Because salt particles make it harder for water particles to freeze back onto the ice, the. Salt makes ice colder because the salt prevents melted water from freezing. This behavior of water makes it possible to. Why Does Salt Melt Ice Fastest.
From lessonlibminimalist.z13.web.core.windows.net
What Makes Ice Melt The Fastest Experiment Why Does Salt Melt Ice Fastest Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. When ice melts, water and ice coexist. Salt makes ice colder because the salt prevents melted water from freezing. Why does salt melt ice? Because salt particles make it harder for water particles to freeze back onto the ice, the.. Why Does Salt Melt Ice Fastest.