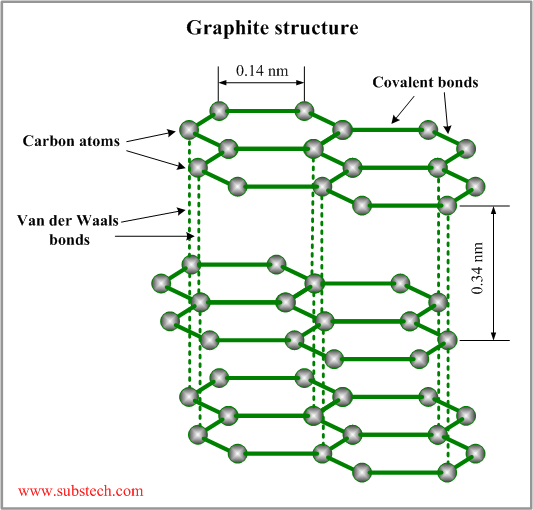
Graphite Definition Chemistry . It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite, mineral consisting of carbon. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite is. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Find out how graphite's properties are. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity.
from socratic.org
Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Graphite, mineral consisting of carbon. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Find out how graphite is. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite's properties are. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered.
What is the chemical formula of graphite? Socratic
Graphite Definition Chemistry Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Graphite, mineral consisting of carbon. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Find out how graphite is. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite's properties are.
From www.youtube.com
Structure of Graphite YouTube Graphite Definition Chemistry Graphite, mineral consisting of carbon. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite's properties are. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. It has a hexagonal lattice of carbon atoms that form sheets, which.. Graphite Definition Chemistry.
From mavink.com
Diagram Of Graphite Structure Graphite Definition Chemistry Graphite, mineral consisting of carbon. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Find out how graphite's properties are. Learn about graphite, a giant covalent structure. Graphite Definition Chemistry.
From en.wikipedia.org
Graphite Wikipedia Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite is used in. Graphite Definition Chemistry.
From socratic.org
What is the chemical formula of graphite? Socratic Graphite Definition Chemistry Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite, mineral consisting of carbon. Find out how graphite's properties are. Graphite is a crystalline form of carbon, known for its unique layered. Graphite Definition Chemistry.
From animalia-life.club
Structure Of Graphite Model Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite. Graphite Definition Chemistry.
From www.dreamstime.com
Model of Graphite Molecular Structure Stock Image Image of chemistry Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. It has a hexagonal lattice of carbon atoms that form sheets, which. Learn about graphite, a giant covalent structure. Graphite Definition Chemistry.
From mwi-inc.com
structure of graphite MWI, Inc. Graphite Definition Chemistry Find out how graphite is. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. It has a hexagonal lattice of carbon atoms that form sheets, which. Learn about graphite, a giant covalent structure with. Graphite Definition Chemistry.
From pixy.org
Graphite structure in chemistry free image download Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Find out how graphite's properties are. Find out how graphite is. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Learn about graphite, a giant covalent structure with. Graphite Definition Chemistry.
From www.savemyexams.com
Graphite structure AQA GCSE Chemistry Revision Notes 2018 Graphite Definition Chemistry Find out how graphite is. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite's properties are. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Graphite, mineral consisting of carbon. Graphite has a greasy feel. Graphite Definition Chemistry.
From www.animalia-life.club
Graphite Atom Structure Graphite Definition Chemistry Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Graphite, mineral consisting of carbon. Find out how graphite's properties are. It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite Definition Chemistry.
From grapheneleaderscanada.com
Graphite and Graphene Graphene Leaders Canada Graphite Definition Chemistry Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Learn about graphite, a form of carbon with hexagonal layers of. Graphite Definition Chemistry.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Graphite Definition Chemistry Graphite, mineral consisting of carbon. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Find out how graphite's properties are. It has a hexagonal lattice of. Graphite Definition Chemistry.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID9350982 Graphite Definition Chemistry Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite is. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite, mineral consisting of carbon. It has a hexagonal lattice of carbon atoms that form sheets,. Graphite Definition Chemistry.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID2755080 Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Learn about graphite, a giant covalent structure with each carbon atom. Graphite Definition Chemistry.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID2654690 Graphite Definition Chemistry Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent. Graphite Definition Chemistry.
From www.researchgate.net
Structure of graphite. Download Scientific Diagram Graphite Definition Chemistry Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Find out how graphite is. Graphite, mineral consisting of carbon. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. It has a hexagonal lattice of. Graphite Definition Chemistry.
From brainly.in
Draw and describe the structure of graphite. Also give some of its Graphite Definition Chemistry It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Find out how graphite's properties are. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Graphite, mineral. Graphite Definition Chemistry.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Graphite Definition Chemistry It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite is. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite has a greasy. Graphite Definition Chemistry.
From parcoscientific.com
Graphite Molecular Model Molecular Models Chemistry Graphite Definition Chemistry Graphite, mineral consisting of carbon. Find out how graphite is. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Find out how graphite's properties are. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Graphite has a greasy feel and. Graphite Definition Chemistry.
From www.savemyexams.com
Giant Covalent Structures HL IB Chemistry Revision Notes 2025 Save Graphite Definition Chemistry Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. It has a hexagonal lattice of carbon atoms that form sheets, which. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite has a greasy feel and leaves a black mark, thus. Graphite Definition Chemistry.
From thechemistrynotes.com
Graphite Structure, Types, Properties, Applications Graphite Definition Chemistry Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical. Graphite Definition Chemistry.
From www.pinterest.com
Covalent Structure Of Graphite Graphite, Molecules, Molecular Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite is. Find out how graphite's properties are. Learn about graphite, a form of carbon with hexagonal layers of atoms, and. Graphite Definition Chemistry.
From www.techno-science.net
Graphite Définition et Explications Graphite Definition Chemistry Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite, mineral consisting of carbon. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical. Graphite Definition Chemistry.
From glossary.periodni.com
Graphite Chemistry Dictionary & Glossary Graphite Definition Chemistry Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Graphite, mineral consisting of carbon. It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite's. Graphite Definition Chemistry.
From sciencenotes.org
What Is an Allotrope? Definition and Examples in Chemistry Graphite Definition Chemistry Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic. Graphite Definition Chemistry.
From superiorgraphite.com
About Graphite Graphite Products Graphite Definition Chemistry Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Graphite, mineral consisting of carbon. Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties.. Graphite Definition Chemistry.
From byjus.com
Describe the structure of Graphite. Graphite Definition Chemistry Find out how graphite is used in electrochemistry, lubrication, and other fields due to its electrical, thermal, and mechanical properties. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite, mineral consisting of carbon. Learn about graphite, a giant. Graphite Definition Chemistry.
From byjus.com
Graphite Definition, Applications, Structures & Properties with Videos Graphite Definition Chemistry Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is. Find out how graphite's properties are. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Graphite. Graphite Definition Chemistry.
From chemifos.blogspot.com
Chemical Composition Of Graphite Chemical Info Graphite Definition Chemistry Find out how graphite is. It has a hexagonal lattice of carbon atoms that form sheets, which. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Find out how graphite's properties are. Graphite. Graphite Definition Chemistry.
From connect.learnpad.com
Graphite structure Content ClassConnect Graphite Definition Chemistry Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite, mineral consisting of carbon. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Graphite is a crystalline form of. Graphite Definition Chemistry.
From www.youtube.com
STRUCTURE, PROPERTIES & USES OF GRAPHITE YouTube Graphite Definition Chemistry Find out how graphite's properties are. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Find out how graphite is. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Learn about graphite, a giant covalent structure with. Graphite Definition Chemistry.
From www.shalom-education.com
Graphite GCSE Chemistry Revision Graphite Definition Chemistry Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its. Graphite Definition Chemistry.
From www.nisenet.org
Scientific Image Graphite models NISE Network Graphite Definition Chemistry Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite, mineral consisting of carbon.. Graphite Definition Chemistry.
From www.youtube.com
Structure of Graphite, Chemistry Lecture Sabaq.pk YouTube Graphite Definition Chemistry It has a hexagonal lattice of carbon atoms that form sheets, which. Graphite is a crystalline form of carbon, known for its unique layered structure and excellent electrical conductivity. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered. Find out how graphite is. Find out. Graphite Definition Chemistry.
From jinsuncarbon.com
What is the chemical formula of graphite? Jinsun Carbon Graphite Definition Chemistry Learn about graphite, a giant covalent structure with each carbon atom forming three covalent bonds with other carbon atoms. Learn about graphite, a form of carbon with hexagonal layers of atoms, and its natural and synthetic varieties. Graphite, mineral consisting of carbon. It has a hexagonal lattice of carbon atoms that form sheets, which. Find out how graphite is used. Graphite Definition Chemistry.