Toothbrush Medical Device . In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification.
from www.philips.com.hk
A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device.
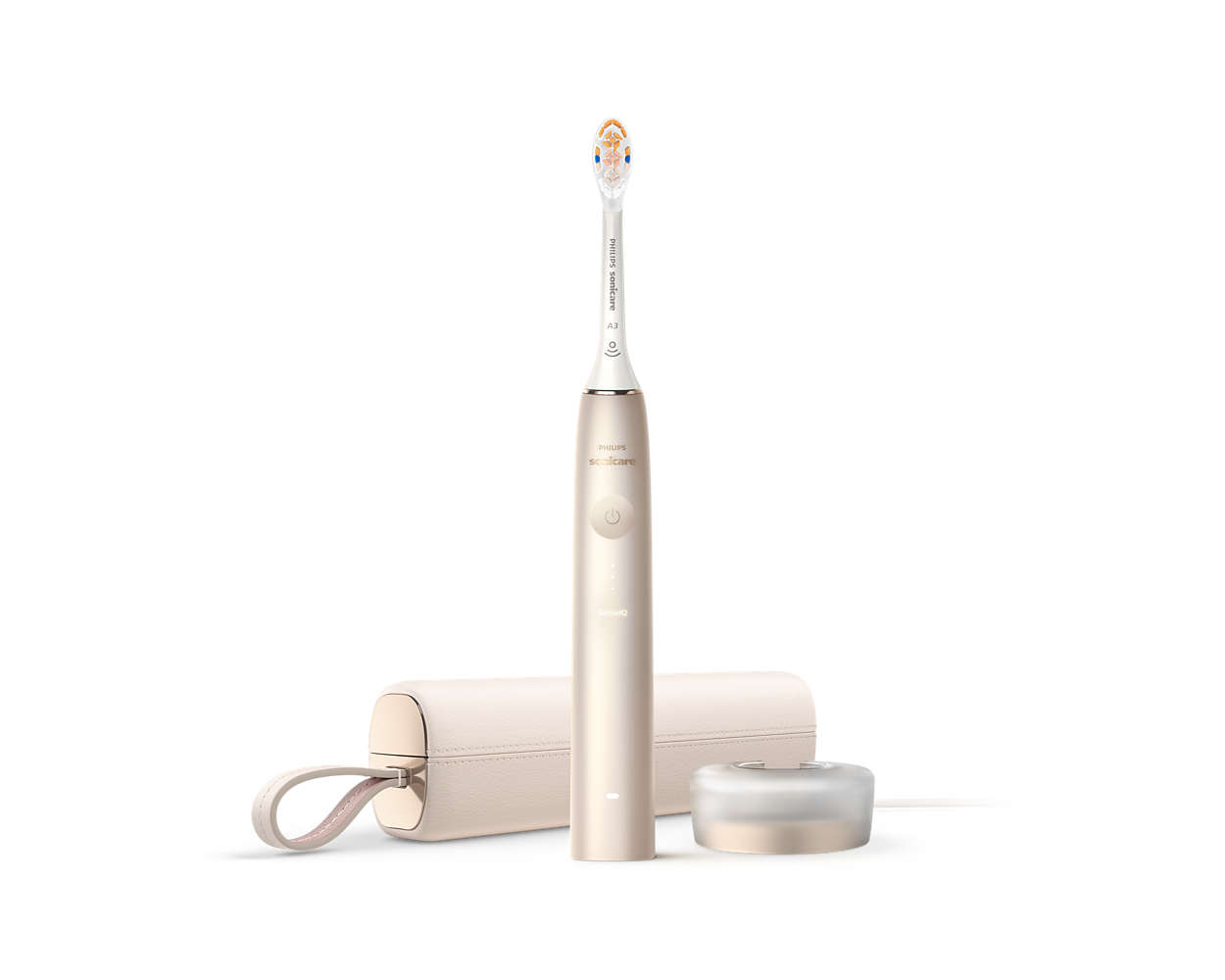
Sonicare 9900 Prestige Power Toothbrush with SenseIQ HX9996/11 Philips
Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device.
From www.dca-design.com
Sensodyne expert toothbrush DCA Design International Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.philips.com.au
DiamondClean 9000 Sonic electric toothbrush with app HX9912/17 Philips Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at. Toothbrush Medical Device.
From www.philips.co.uk
DiamondClean 9000 Sonic electric toothbrush with app HX9914/55 Philips Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.philips.com.hk
DiamondClean 9000 Sonic electric toothbrush with app HX9912/07 Philips Toothbrush Medical Device A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual. Toothbrush Medical Device.
From www.philips.ca
DiamondClean Sonic electric toothbrush HX9354/75 Sonicare Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at. Toothbrush Medical Device.
From www.philips.com.au
S Sensitive Standard sonic toothbrush heads HX6053/63 Sonicare Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k). Toothbrush Medical Device.
From mcreatmedical.en.made-in-china.com
Medical Device Disposable Dental Sponge Oral Toothbrush with Suction Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.philips.com.hk
Sonicare 9900 Prestige Power Toothbrush with SenseIQ HX9996/11 Philips Toothbrush Medical Device A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual. Toothbrush Medical Device.
From www.philips.co.nz
DiamondClean Sonic electric toothbrush HX9368/35 Sonicare Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.aliexpress.com
Disposable Medical Sponge Toothbrush ICU suction swab Oral Care Single Toothbrush Medical Device A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.nsmedicaldevices.com
ColgatePalmolive launches new smart electric toothbrush hum by Colgate Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.superdentalstore.com
Germ Terminator Toothbrush Sanitizer Super Dental Store Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual. Toothbrush Medical Device.
From www.medical4u.ie
Curaprox Orthodontic Ultra Soft Toothbrush (Blister) Medical4U Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual. Toothbrush Medical Device.
From www.dreamstime.com
Close Up Medical Device, Toothbrush with White Toothpaste Isolated on Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual. Toothbrush Medical Device.
From www.usa.philips.com
DiamondClean Smart Sonic electric toothbrush with app HX9903/31 Sonicare Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.philips.ca
DailyClean 1100 Sonic electric toothbrush HX3411/05 Sonicare Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual. Toothbrush Medical Device.
From www.desertcart.ae
Family UV Toothbrush Holder & Sanitizer, FDA Listed Medical Device Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at. Toothbrush Medical Device.
From www.pinterest.com
SEAGO Original Family Kits of Three Sonic Toothbrush UV Sterilizer Toothbrush Medical Device A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.philips.com.sg
DiamondClean Smart Sonic electric toothbrush with app HX9924/46 Sonicare Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.usa.philips.com
ExpertClean 7400 Sonic electric toothbrush with app HX9685/03 Sonicare Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual. Toothbrush Medical Device.
From home.bt.com
Amabrus The automatic toothbrush that cleans your teeth in 10 seconds BT Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.usa.philips.com
FlexCare Platinum Connected Bluetooth® connected toothbrushDispense Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.philips.co.uk
DiamondClean 9000 Sonic electric toothbrush with app HX9911/53 Philips Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual. Toothbrush Medical Device.
From www.youtube.com
Healthcare Toothbrush a smart toothbrush that monitors health YouTube Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.dreamstime.com
Dental Oral Care Kit Multicolor Toothbrushes at Medical Exhibition Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at. Toothbrush Medical Device.
From www.usa.philips.com
eSeries Standard sonic toothbrush heads HX7002/60 Sonicare Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.usa.philips.com
ExpertClean 7500 Sonic electric toothbrush with app HX9690/05 Philips Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual. Toothbrush Medical Device.
From www.philips.com.sg
DiamondClean 9000 Series Power Toothbrush Special Edition HX9911/73 Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.philips.ca
ProtectiveClean 5100 Sonic electric toothbrush HX6877/73 Sonicare Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k). Toothbrush Medical Device.
From alohamedicalhk.com
OralClean G100 electric suction toothbrush Aloha Medical Limted 亞諾醫療 Toothbrush Medical Device A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at. Toothbrush Medical Device.
From www.philips.com.kw
Sonicare 9900 Prestige Power Toothbrush with SenseIQ HX9992/22 Philips Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. In this example, a manual. Toothbrush Medical Device.
From www.philips.co.uk
G3 Premium Gum Care Standard sonic toothbrush heads HX9052/17 Sonicare Toothbrush Medical Device The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.pinterest.com
Philips Sonicare PowerUp battery toothbrush Toothbrush design Toothbrush Medical Device A manual toothbrush is 510(k) exempt, meaning it does not require premarket. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. In this example, a manual toothbrush is a class 1 medical device. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.
From www.usa.philips.com
Philips One by Sonicare Battery Toothbrush HY1100/03 Philips Toothbrush Medical Device A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. The medical device regulation (mdr), applicable from 26 may 2021, has resulted in various changes with regard to classification. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k). Toothbrush Medical Device.
From www.philips.co.uk
DiamondClean 9000 Sonic electric toothbrush with app HX9914/57 Philips Toothbrush Medical Device In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is a device composed of a shaft with either natural or synthetic bristles at one end intended to remove adherent plaque. A manual toothbrush is 510(k) exempt, meaning it does not require premarket. The medical device regulation (mdr), applicable from 26 may 2021, has resulted. Toothbrush Medical Device.