Medical Devices Service Canada . Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Also search for a licensed device using. Access forms and guidance documents to help you apply for a medical device licence. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Health canada's website has a section for medical device regulations:
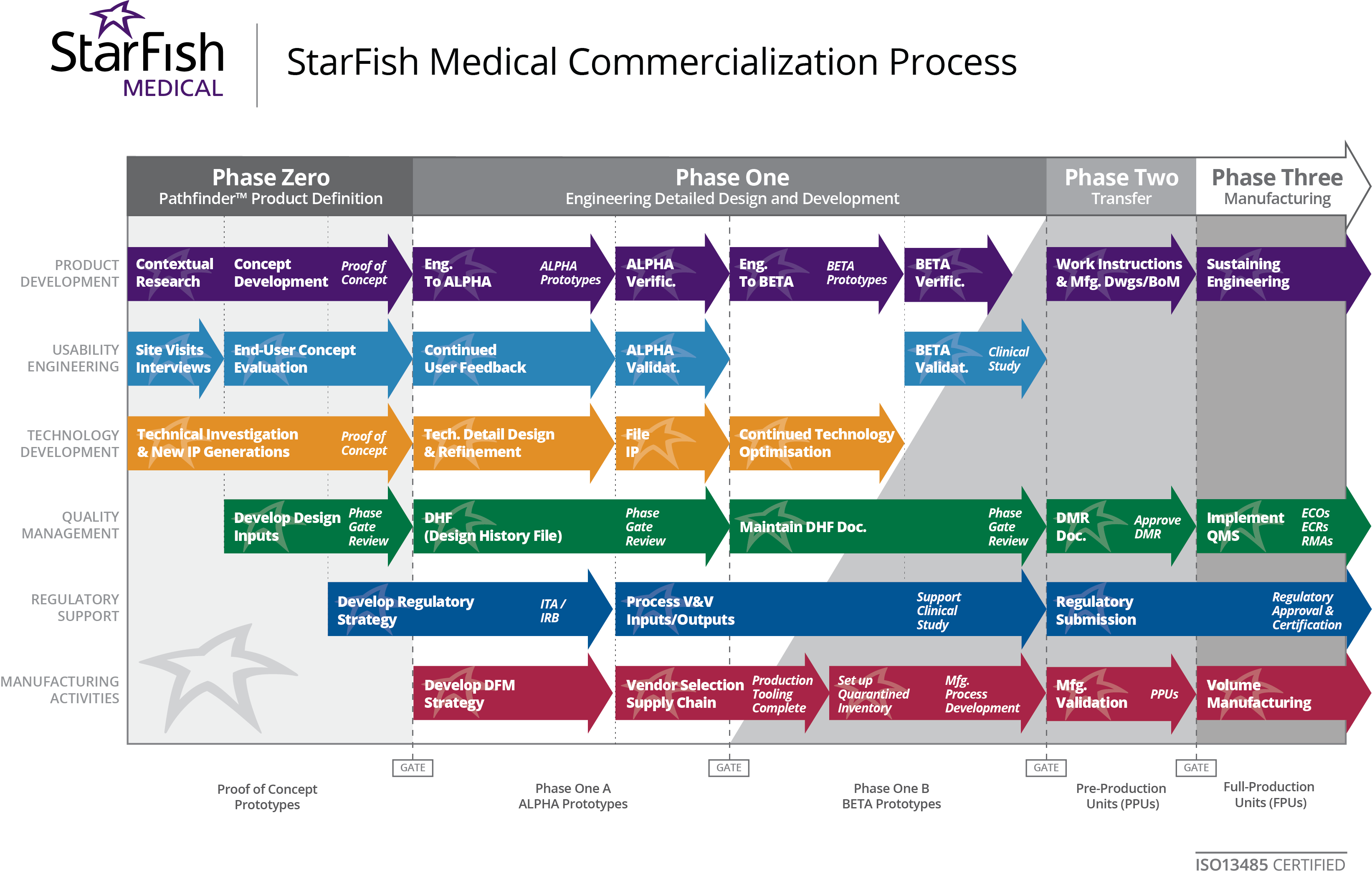
from starfishmedical.com
Also search for a licensed device using. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the new medical devices licence history archive search. Access forms and guidance documents to help you apply for a medical device licence. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians.
Medical device contract manufacturing StarFish Medical
Medical Devices Service Canada Access forms and guidance documents to help you apply for a medical device licence. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Access forms and guidance documents to help you apply for a medical device licence. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Also search for a licensed device using. Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the new medical devices licence history archive search. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Devices Service Canada Access forms and guidance documents to help you apply for a medical device licence. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Also search for a licensed device using. Selecting the archived licence search link takes you to the new medical devices licence history archive. Medical Devices Service Canada.
From www.canada.ca
Frequently asked questions Medical device establishment licensing and Medical Devices Service Canada Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the new medical devices licence history archive search. Also search for a licensed device using. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. This system has. Medical Devices Service Canada.
From www.joharidigital.com
Health Canada Approval Process for Medical Devices StepbyStep Guide Medical Devices Service Canada Health canada's website has a section for medical device regulations: Also search for a licensed device using. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Access forms and guidance documents to help you apply for a medical device licence. Defines medical devices,. Medical Devices Service Canada.
From www.fortdefianceind.com
Canada P2131 Maintenance Training Fort Defiance Industries LLC Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to. Medical Devices Service Canada.
From www.regdesk.co
Health Canada Guidance on Private Label Medical Devices RegDesk Medical Devices Service Canada This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems,. Medical Devices Service Canada.
From emmainternational.com
Health Canada’s Special Access Program for Medical Devices Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Access forms and guidance documents to help you apply for a medical device licence. Also search for a licensed device using. Health canada's website has a section for medical device regulations: Provides access to the health canada medical devices active licence. Medical Devices Service Canada.
From www.castoredc.com
Medical Device Regulation 4 ways it will impact your device studies Medical Devices Service Canada This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Also search for a licensed device using. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Health canada's website has a section for medical device regulations:. Medical Devices Service Canada.
From omcmedical.com
Health Canada Medical Device Listing OMC Medical Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. Access forms and guidance documents to help you apply for a medical device licence. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Also search for a licensed device. Medical Devices Service Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Devices Service Canada Access forms and guidance documents to help you apply for a medical device licence. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Health. Medical Devices Service Canada.
From www.thema-med.com
Health Canada new PMS requirements for Medical Devices Medical Devices Service Canada Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Defines. Medical Devices Service Canada.
From mtelehealth.com
mTelehealth Presents the Telehealth Home Health and Remote Patient Medical Devices Service Canada Also search for a licensed device using. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Defines medical devices, how they are licensed and regulated, types of licences, monitoring. Medical Devices Service Canada.
From www.celegence.com
Medical Devices Services and Solutions Celegence Medical Devices Service Canada Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to. Medical Devices Service Canada.
From www.infoway-inforoute.ca
Infographic The Diffusion of Smart Devices for Health in Canada Medical Devices Service Canada Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Access forms and guidance documents to help you apply for a medical device licence. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Selecting the archived licence. Medical Devices Service Canada.
From mobilityforesights.com
Canada Medical Devices Market 20222027 January 2024 Updated Medical Devices Service Canada Health canada's website has a section for medical device regulations: This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Selecting the archived licence search. Medical Devices Service Canada.
From medicaldeviceacademy.com
Canadian Device Licensing inar Live on May 24, 2017 Medical Device Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. Health canada's website has a section for medical device regulations: Also search for a licensed device using. Access forms and guidance documents to help you apply for a medical device licence. Provides access to the health canada medical devices active licence (mdall), a. Medical Devices Service Canada.
From www.verifiedmarketresearch.com
InDepth Industry Outlook Medical Devices Market Size & Forecast Medical Devices Service Canada Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Access forms and guidance documents to help you apply for a medical device licence. Selecting the archived licence search link takes you. Medical Devices Service Canada.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Access forms and guidance documents to help you apply for a medical device licence. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Also search for a licensed device using. Health canada's website. Medical Devices Service Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. Access forms and guidance documents to help you apply for a medical device licence. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Health canada's website. Medical Devices Service Canada.
From cosmereg.com
Canada Medical Device Services & Regulations FDA Clearance Medical Devices Service Canada Also search for a licensed device using. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Access forms and guidance documents to help you apply for a medical device licence. Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the. Medical Devices Service Canada.
From www.lookeetech.com
Understanding Health Canada Medical Device Licensing LOOKEETech Medical Devices Service Canada Also search for a licensed device using. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Defines medical devices, how they are licensed and regulated, types. Medical Devices Service Canada.
From starfishmedical.com
Canadian medical device industry Medical Devices Service Canada Access forms and guidance documents to help you apply for a medical device licence. Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the new medical devices licence history archive search. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians.. Medical Devices Service Canada.
From www.regdesk.co
Consultation on Changes to Canadian Medical Device Regulations RegDesk Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class. Medical Devices Service Canada.
From verifydiagnostics.com
All About Compliance Medical Device Licensing in Canada Verify Medical Devices Service Canada Also search for a licensed device using. Health canada's website has a section for medical device regulations: Access forms and guidance documents to help you apply for a medical device licence. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Selecting the archived licence search link takes you to the. Medical Devices Service Canada.
From www.medline.ca
Medical Device Reprocessing Medline Canada Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the new medical devices licence history archive search. This system has been designed to help health care workers, who are contemplating the. Medical Devices Service Canada.
From www.qandrcanada.com
Medical Device Classification and Submission Q&R Canada Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Also search for a licensed device using. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Health canada's website has a section for medical device regulations: Provides access to the health. Medical Devices Service Canada.
From nthsolutions.co.uk
Medical Equipment Supply and Servicing NTH Solutions Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. This system has been designed to help health care workers, who are contemplating the purchase of a class. Medical Devices Service Canada.
From medicaldevices.freyrsolutions.com
Medical Device Registration Canada, Health Canada, MDSAP certification Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class. Medical Devices Service Canada.
From www.canada.ca
Drug and medical device highlights 2020 Medical devices Canada.ca Medical Devices Service Canada This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Selecting the archived licence search link takes you to the new medical devices licence history. Medical Devices Service Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Devices Service Canada Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Health canada's website has a section for medical device regulations: Access forms and guidance documents. Medical Devices Service Canada.
From www.massdevice.com
An expert's guide to developing medical devices MassDevice Medical Devices Service Canada Defines medical devices, how they are licensed and regulated, types of licences, monitoring after licensing, reporting problems, and health. Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Health canada's website has a section for medical device regulations: Access forms and guidance documents to help you. Medical Devices Service Canada.
From www.youtube.com
How to get Medical Device License in Canada Medical Device Sales in Medical Devices Service Canada Also search for a licensed device using. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Access forms and guidance documents to help you apply for a medical device licence. Selecting the archived licence search link takes you to the new medical devices licence history archive search. Health canada's website. Medical Devices Service Canada.
From www.canada.ca
Approved in 2020 Medical devices Canada.ca Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Health canada's website has a section for medical device regulations: Provides access to the health canada medical devices active licence (mdall), a database of. Medical Devices Service Canada.
From starfishmedical.com
Medical device contract manufacturing StarFish Medical Medical Devices Service Canada Access forms and guidance documents to help you apply for a medical device licence. Medical devices made in canada or abroad must meet high safety and quality standards before being sold to canadians. Health canada's website has a section for medical device regulations: Selecting the archived licence search link takes you to the new medical devices licence history archive search.. Medical Devices Service Canada.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Devices Service Canada Selecting the archived licence search link takes you to the new medical devices licence history archive search. This system has been designed to help health care workers, who are contemplating the purchase of a class ii, iii or iv medical device, to verify. Access forms and guidance documents to help you apply for a medical device licence. Defines medical devices,. Medical Devices Service Canada.
From www.regdesk.co
HSA Guidance on Grouping of Medical Devices Overview RegDesk Medical Devices Service Canada Provides access to the health canada medical devices active licence (mdall), a database of all licensed class ii, iii, and iv medical devices. Health canada's website has a section for medical device regulations: Access forms and guidance documents to help you apply for a medical device licence. Medical devices made in canada or abroad must meet high safety and quality. Medical Devices Service Canada.