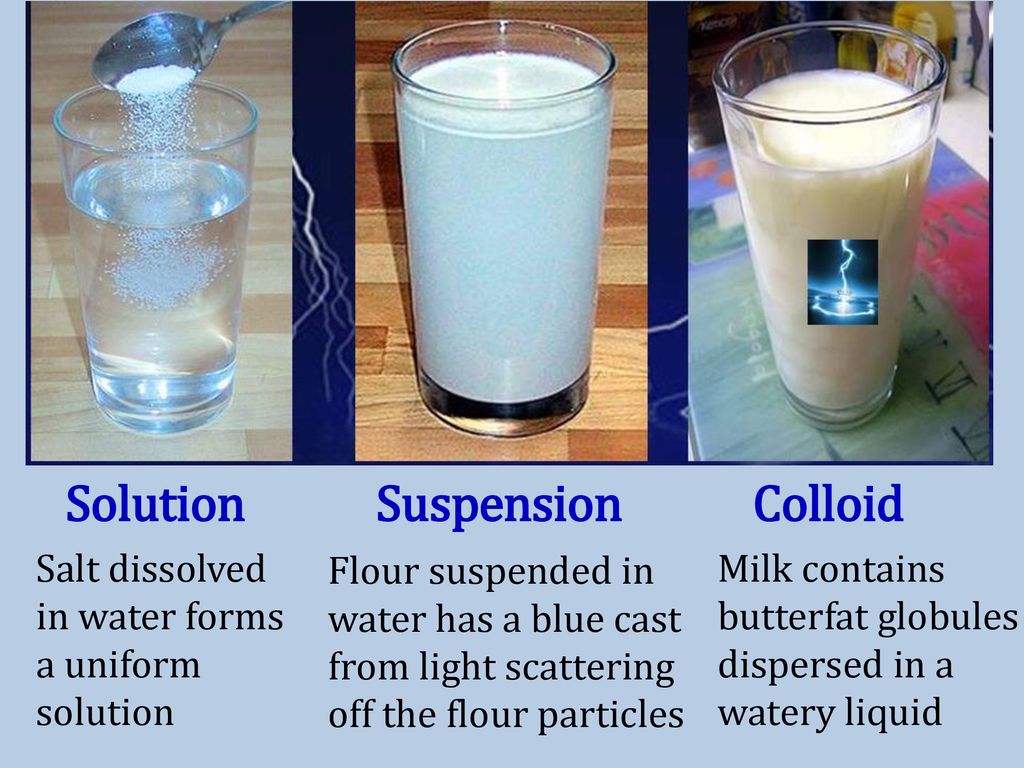
Mixture Examples Of Suspension . In order to be a suspension, the particles must not dissolve in the fluid. Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. Examples include oil in water and dust in the air. Everyday examples of suspensions include mud, flour in. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture that settles out upon standing. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles.
from
In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. Everyday examples of suspensions include mud, flour in. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Examples include oil in water and dust in the air. In order to be a suspension, the particles must not dissolve in the fluid. Common suspensions include paint, blood, and hot. A suspension is a heterogeneous mixture that settles out upon standing.
Mixture Examples Of Suspension Examples include oil in water and dust in the air. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Examples include oil in water and dust in the air. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. In order to be a suspension, the particles must not dissolve in the fluid. A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in.
From
Mixture Examples Of Suspension In order to be a suspension, the particles must not dissolve in the fluid. Everyday examples of suspensions include mud, flour in. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Common suspensions include paint, blood, and hot. In chemistry, a suspension it is a type of. Mixture Examples Of Suspension.
From www.slideserve.com
PPT Chapter 13, (14) Mixtures and Aqueous Solutions PowerPoint Mixture Examples Of Suspension Examples include oil in water and dust in the air. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. Everyday examples of suspensions include mud, flour in. Common suspensions include paint, blood, and hot. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. In chemistry, a. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. Everyday examples of suspensions include mud, flour in. Examples include oil in water and dust in the air. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Examples include oil in water and dust in the air. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. In chemistry, a suspension it is a type of heterogeneous mixture formed by. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. Everyday examples of suspensions include mud, flour in. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. In chemistry, a suspension is a mixture in which the. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Common suspensions include paint, blood, and hot. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. A suspension is a heterogeneous mixture that settles out upon standing. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Common suspensions include paint, blood, and hot. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. A suspension is a heterogeneous mixture that settles out upon standing. A suspension is a mixture. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension A suspension is a heterogeneous mixture that settles out upon standing. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. In chemistry, a suspension it. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Everyday examples of suspensions include mud, flour in. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. Examples include oil in water and dust. Mixture Examples Of Suspension.
From www.expii.com
Suspensions — Definition & Examples Expii Mixture Examples Of Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture that settles out upon standing. Examples include oil in water and dust in the air. Everyday examples of suspensions include mud, flour in. In chemistry, a suspension is a mixture in which. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Everyday examples of suspensions include mud, flour in. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In order to be a suspension, the particles must not dissolve in the fluid. Examples include oil in water and dust in the air. In chemistry, a suspension is a mixture in which the solute particles do not. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. The suspension is a heterogeneous. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Examples include oil in water and dust in the air. A suspension is a heterogeneous mixture that settles out upon standing. Common suspensions include paint, blood, and hot. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. A colloid is a heterogeneous mixture in which the. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. Everyday examples of suspensions include mud, flour in. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture that settles out upon standing. The suspension is a heterogeneous. Mixture Examples Of Suspension.
From alani-jolpblogjordan.blogspot.com
A Mixture That Separates Into Layers Upon Standing Is a Mixture Examples Of Suspension Examples include oil in water and dust in the air. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In order to be a suspension, the particles must not dissolve in the fluid. In chemistry, a suspension it is a type of heterogeneous mixture formed by one. Mixture Examples Of Suspension.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Mixture Examples Of Suspension Everyday examples of suspensions include mud, flour in. Common suspensions include paint, blood, and hot. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a.. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. A colloid. Mixture Examples Of Suspension.
From pt.slideshare.net
Solutions, suspensions, and colloids Mixture Examples Of Suspension In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in. The suspension is a heterogeneous mixture in which. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Examples include oil in water and dust in the air. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. The suspension is a heterogeneous mixture in which the solute particles do not. Mixture Examples Of Suspension.
From www.sliderbase.com
Properties of Water Presentation Biology Mixture Examples Of Suspension In chemistry, a suspension is a mixture in which the solute particles do not dissolve. In order to be a suspension, the particles must not dissolve in the fluid. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. A suspension is a mixture of particles with diameters of about 1. Mixture Examples Of Suspension.
From kunduz.com
Homogeneous Mixtures Definition, Properties, Types, Examples Mixture Examples Of Suspension Examples include oil in water and dust in the air. A suspension is a heterogeneous mixture that settles out upon standing. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the bulk of the medium. Common suspensions include paint, blood, and hot. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. In chemistry, a suspension is a mixture in. Mixture Examples Of Suspension.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Mixture Examples Of Suspension In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. Everyday examples of suspensions include mud, flour in. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. Common suspensions include paint, blood, and hot. A suspension is a mixture of particles with diameters of about 1. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. Everyday examples of suspensions include mud, flour in. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. The suspension is a heterogeneous mixture in which the solute particles do not dissolve but get suspended throughout the. Mixture Examples Of Suspension.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets Mixture Examples Of Suspension In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. Examples include oil in water and dust in the air. Everyday examples of suspensions include mud, flour in. Common. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Common suspensions include paint, blood, and hot. In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout. Mixture Examples Of Suspension.
From brainly.ph
what is a suspension mixture Brainly.ph Mixture Examples Of Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Common suspensions include paint, blood, and hot. Examples include oil in water and dust in the air. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In chemistry, a suspension it is a. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In order to be a suspension, the particles must not dissolve in the fluid. Examples include oil in water and dust in the air. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. The suspension is a heterogeneous mixture in which the solute particles do not dissolve. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. Examples include oil in water and dust in the air. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and. Mixture Examples Of Suspension.
From www.researchgate.net
Examples of suspension beverages. Download Scientific Diagram Mixture Examples Of Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. In. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Everyday examples of suspensions include mud, flour in. Examples include oil in water and dust in the air. Common suspensions include paint, blood, and hot. A suspension is a heterogeneous mixture that settles out upon standing. In chemistry,. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In order to be a suspension, the particles must not dissolve in the fluid. In chemistry, a suspension is a heterogeneous mixture of a fluid and solid particles. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. A suspension is a heterogeneous mixture that settles out upon. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension In chemistry, a suspension it is a type of heterogeneous mixture formed by one or more substances in solid. In order to be a suspension, the particles must not dissolve in the fluid. Everyday examples of suspensions include mud, flour in. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Mixture Examples Of Suspension.
From
Mixture Examples Of Suspension Everyday examples of suspensions include mud, flour in. A suspension is a heterogeneous mixture that settles out upon standing. In chemistry, a suspension is a mixture in which the solute particles do not dissolve. A suspension is a mixture of particles with diameters of about 1 µm (1000 nm) that are distributed throughout a second phase. The suspension is a. Mixture Examples Of Suspension.