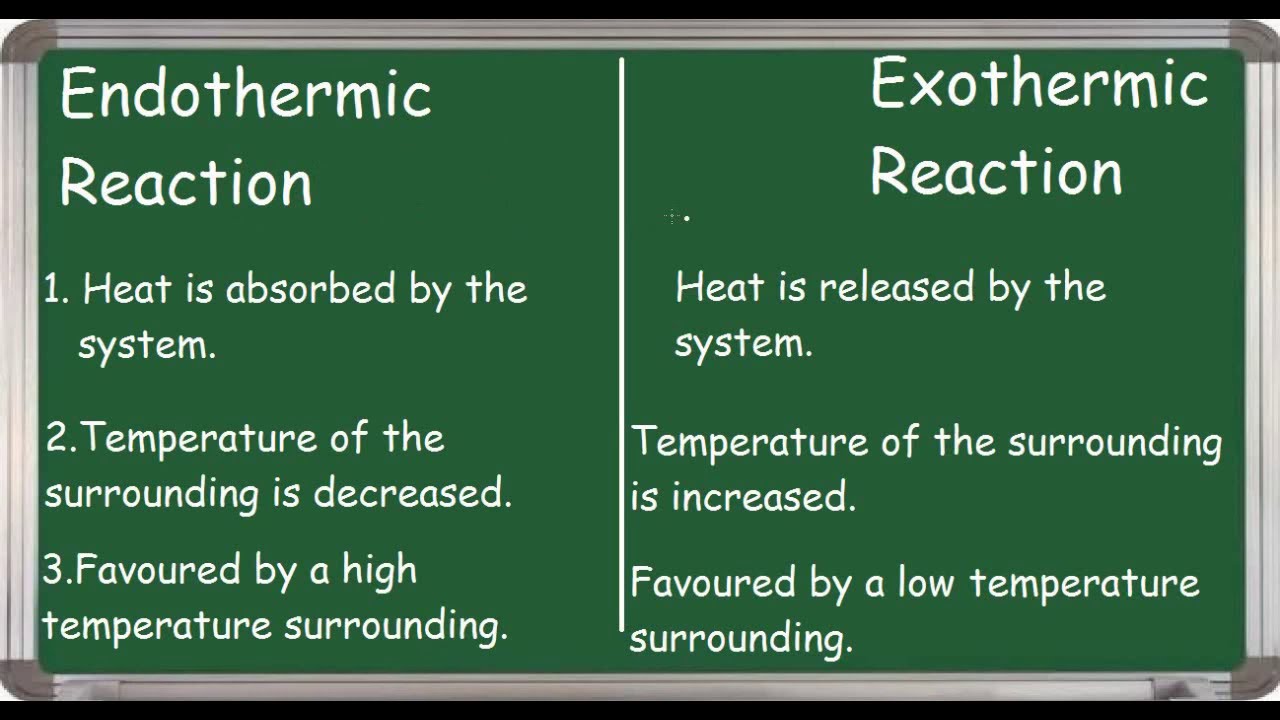
Endothermic Reaction This . These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Some examples of endothermic reactions are: Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. I am assuming that you have already read the page introducing energy changes during. An endothermic reaction is one in which heat is absorbed. The reaction between ethanoic acid and sodium carbonate. These changes in energy can either cause a reaction to use up or to produce heat. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. When chemical reactions happen, there is a change in energy.
from khatiar1955a.altervista.org
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic reaction is one in which heat is absorbed. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. When chemical reactions happen, there is a change in energy. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. I am assuming that you have already read the page introducing energy changes during. These changes in energy can either cause a reaction to use up or to produce heat.
The Difference Between Endothermic and Exothermic Reactions
Endothermic Reaction This These changes in energy can either cause a reaction to use up or to produce heat. The reaction between ethanoic acid and sodium carbonate. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When chemical reactions happen, there is a change in energy. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. I am assuming that you have already read the page introducing energy changes during. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These changes in energy can either cause a reaction to use up or to produce heat. Some examples of endothermic reactions are: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is one in which heat is absorbed. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction This Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is one in which heat is absorbed. I am assuming that you have already read the page introducing energy changes during. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.. Endothermic Reaction This.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction This For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. The reaction between ethanoic. Endothermic Reaction This.
From narodnatribuna.info
Endothermic Reactions Definition And Examples Endothermic Reaction This Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. I am assuming that. Endothermic Reaction This.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction This An endothermic reaction is one in which heat is absorbed. These changes in energy can either cause a reaction to use up or to produce heat. Some examples of endothermic reactions are: Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. I am assuming that you have. Endothermic Reaction This.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction This I am assuming that you have already read the page introducing energy changes during. These changes in energy can either cause a reaction to use up or to produce heat. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The reaction between ethanoic acid and sodium carbonate. Revise and understand what. Endothermic Reaction This.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction This These changes in energy can either cause a reaction to use up or to produce heat. An endothermic reaction is one in which heat is absorbed. I am assuming that you have already read the page introducing energy changes during. Some examples of endothermic reactions are: When chemical reactions happen, there is a change in energy. For an endothermic chemical. Endothermic Reaction This.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction This These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Some examples of endothermic reactions are: When chemical reactions happen, there is a change in energy. An endothermic reaction is one in which heat is absorbed. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. Endothermic Reaction This.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction This Some examples of endothermic reactions are: Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. I am assuming that you have already read the page introducing energy changes during. The reaction between ethanoic acid and sodium carbonate. Endothermic reactions are chemical reactions in which the reactants absorb. Endothermic Reaction This.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction This I am assuming that you have already read the page introducing energy changes during. These changes in energy can either cause a reaction to use up or to produce heat. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Some examples of endothermic reactions are: An endothermic reaction is one in which heat is absorbed.. Endothermic Reaction This.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction This These changes in energy can either cause a reaction to use up or to produce heat. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Endothermic reactions are chemical reactions in which the reactants absorb. Endothermic Reaction This.
From quizlet.com
Year 10 Chemistry Exothermic and Endothermic Reactions Diagram Endothermic Reaction This Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Some examples of endothermic reactions are: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Exothermic reactions transfer energy to the surroundings and the temperature of the. Endothermic Reaction This.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction This Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These changes in energy can either cause a reaction to use up or to produce heat. I am assuming that you have already read the page introducing energy changes during. Revise and understand what endothermic and exothermic reactions are and how the. Endothermic Reaction This.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction This Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. When chemical reactions happen, there is a change in energy. I am assuming that you have already read the page introducing energy changes during. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings.. Endothermic Reaction This.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction This The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is one in which heat is absorbed. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Some examples of endothermic reactions are: These changes in energy can either cause a reaction to use up or to produce heat. Revise and. Endothermic Reaction This.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction This Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When chemical reactions happen, there is a change in energy. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Some examples of endothermic reactions are: These changes in energy can either cause a. Endothermic Reaction This.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Reaction This Some examples of endothermic reactions are: These changes in energy can either cause a reaction to use up or to produce heat. The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Endothermic Reaction This.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction This The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is one in which heat is absorbed. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. For an endothermic chemical reaction to. Endothermic Reaction This.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction This Some examples of endothermic reactions are: These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. These changes in energy can either cause a reaction to use up or to produce heat. I am assuming that you have already read the page introducing energy changes during. An endothermic reaction is one in which heat is absorbed.. Endothermic Reaction This.
From h-o-m-e.org
Chilling with Endothermic Reactions Endothermic Reaction This The reaction between ethanoic acid and sodium carbonate. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When chemical reactions happen, there is a change in energy. I am assuming that you have already read the page introducing energy changes during. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.. Endothermic Reaction This.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction This Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Some examples of endothermic reactions are: These changes in energy can either cause a reaction to use up or to produce heat. The reaction between ethanoic acid and sodium carbonate. Exothermic reactions transfer energy to the surroundings and the temperature of the. Endothermic Reaction This.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Endothermic Reaction This Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. When chemical reactions happen, there is a change in energy. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Some examples of endothermic reactions are: The reaction between ethanoic. Endothermic Reaction This.
From klamequqc.blob.core.windows.net
Endothermic Reaction Used In A Sentence at Terrence Rankin blog Endothermic Reaction This For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. I am assuming that you have already read the page introducing energy changes during. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These changes in energy can either cause a. Endothermic Reaction This.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction This These changes in energy can either cause a reaction to use up or to produce heat. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Endothermic Reaction This.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction This Some examples of endothermic reactions are: When chemical reactions happen, there is a change in energy. I am assuming that you have already read the page introducing energy changes during. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is one in which heat is absorbed. Exothermic reactions transfer energy to the surroundings and the temperature of the. Endothermic Reaction This.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction This Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. An endothermic reaction is one in which heat is absorbed. When chemical. Endothermic Reaction This.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction This These changes in energy can either cause a reaction to use up or to produce heat. I am assuming that you have already read the page introducing energy changes during. An endothermic reaction is one in which heat is absorbed. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.. Endothermic Reaction This.
From www.youtube.com
Endothermic Reactions YouTube Endothermic Reaction This I am assuming that you have already read the page introducing energy changes during. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. These changes in energy can either cause a reaction to use up or to produce heat. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy. Endothermic Reaction This.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Endothermic Reaction This I am assuming that you have already read the page introducing energy changes during. Some examples of endothermic reactions are: Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.. Endothermic Reaction This.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Reaction This Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. These changes in energy can either cause a reaction to use up or to produce heat. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. For an endothermic chemical reaction to proceed, the reactants must absorb energy. Endothermic Reaction This.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction This Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. I am assuming that you have already read the page introducing energy changes during. Some examples of endothermic reactions are: These changes in energy can either cause a reaction to use up or to produce heat. The reaction between ethanoic acid and sodium carbonate. These reactions. Endothermic Reaction This.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Reaction This The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. When chemical reactions happen, there is a change in energy. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Exothermic reactions. Endothermic Reaction This.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction This Some examples of endothermic reactions are: For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. These changes in energy can either cause a reaction to use up or to produce heat. Revise and understand what. Endothermic Reaction This.
From facts.net
16 Intriguing Facts About Endothermic Endothermic Reaction This I am assuming that you have already read the page introducing energy changes during. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When chemical reactions happen, there is a change in energy. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These changes. Endothermic Reaction This.
From mavink.com
Draw An Energy Level Diagram For An Endothermic Reaction Endothermic Reaction This The reaction between ethanoic acid and sodium carbonate. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. I am assuming that you have already read the page introducing energy changes during. Revise and understand what. Endothermic Reaction This.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction This The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. These reactions lower the temperature of their surrounding area, thereby. Endothermic Reaction This.