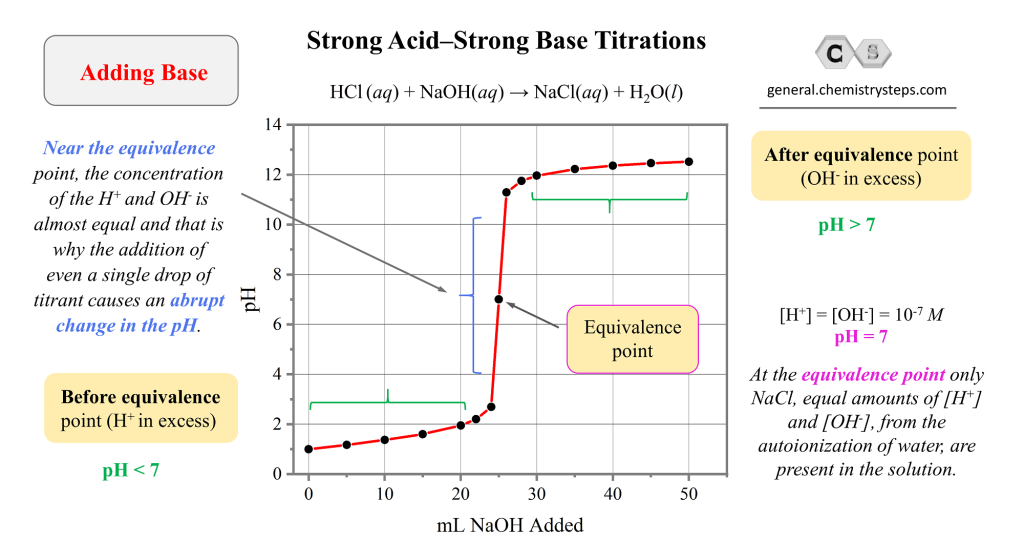
Titration Curve Monoprotic Or Polyprotic . The way you normally carry out a titration involves adding the acid to the alkali. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Polyprotic acids are acids that can lose several protons per molecule. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. A summary of the important curves. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Titrations of polyprotic acids have more than. Therefore, it varies from 0 to 1 for an acid that releases one proton. The first marker is if the initial ph is above or below 7. Key features of titration curves: Here are reduced versions of.
from general.chemistrysteps.com
Therefore, it varies from 0 to 1 for an acid that releases one proton. Here are reduced versions of. A summary of the important curves. Polyprotic acids are acids that can lose several protons per molecule. The first marker is if the initial ph is above or below 7. Key features of titration curves: Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. The way you normally carry out a titration involves adding the acid to the alkali.
Strong AcidStrong Base Titrations Chemistry Steps
Titration Curve Monoprotic Or Polyprotic Key features of titration curves: The way you normally carry out a titration involves adding the acid to the alkali. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. A summary of the important curves. Therefore, it varies from 0 to 1 for an acid that releases one proton. Key features of titration curves: Polyprotic acids are acids that can lose several protons per molecule. The first marker is if the initial ph is above or below 7. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Titrations of polyprotic acids have more than. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Here are reduced versions of.
From www.numerade.com
SOLVED Model Titration Curves involving Polyprotic acids Oxalic acid Titration Curve Monoprotic Or Polyprotic An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. The first marker is if the initial ph is above or below 7. Titrations of polyprotic acids have more than. A summary of the important curves. Key features of titration curves: However, a polyprotic acid differs from a monoprotic acid because it has more than. Titration Curve Monoprotic Or Polyprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Monoprotic Or Polyprotic Titrations of polyprotic acids have more than. A summary of the important curves. Polyprotic acids are acids that can lose several protons per molecule. The first marker is if the initial ph is above or below 7. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Therefore, it varies from 0 to 1. Titration Curve Monoprotic Or Polyprotic.
From www.vrogue.co
What Is Titration See Titration Curves Polyprotic Aci vrogue.co Titration Curve Monoprotic Or Polyprotic Here are reduced versions of. Key features of titration curves: Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. They can be further categorized. Titration Curve Monoprotic Or Polyprotic.
From solvedlib.com
Titration Curves of Unknown Monoprotic Acids1Volume N… SolvedLib Titration Curve Monoprotic Or Polyprotic Polyprotic acids are acids that can lose several protons per molecule. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Therefore, it varies from 0 to 1 for an acid that releases one proton. The first marker is if the initial ph is. Titration Curve Monoprotic Or Polyprotic.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Monoprotic Or Polyprotic A summary of the important curves. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Key features of titration curves: Titrations of polyprotic acids have more than. The first marker is if the initial ph is above or below 7. The way you normally carry out a titration involves adding. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Titration Curve Monoprotic Or Polyprotic They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Polyprotic acids are acids that can lose several protons per molecule. A summary of the important curves. Titrations of polyprotic acids have more than. Key features of titration curves: However, a polyprotic acid differs from a monoprotic acid because it has more than one. Titration Curve Monoprotic Or Polyprotic.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Monoprotic Or Polyprotic An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Titrations of polyprotic acids have more than. Key features of titration curves: A summary of the important curves. The first marker is if the initial ph is above or. Titration Curve Monoprotic Or Polyprotic.
From mavink.com
Monoprotic Acid Titration Curve Titration Curve Monoprotic Or Polyprotic The way you normally carry out a titration involves adding the acid to the alkali. Key features of titration curves: They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Therefore, it varies from 0 to 1 for an acid that releases one proton. The first marker is if the initial ph is above. Titration Curve Monoprotic Or Polyprotic.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Monoprotic Or Polyprotic The way you normally carry out a titration involves adding the acid to the alkali. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Polyprotic acids are acids that can lose several protons per molecule. A summary of the important curves. An arrhenius acid donates a proton (h+ h +),. Titration Curve Monoprotic Or Polyprotic.
From mungfali.com
Polyprotic Acid Titration Curve Titration Curve Monoprotic Or Polyprotic A summary of the important curves. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. The way you normally carry out a titration involves adding the acid to the alkali. Titrations of polyprotic acids have more than. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+. Titration Curve Monoprotic Or Polyprotic.
From quizlet.com
Draw a titration curve for a polyprotic acid. Quizlet Titration Curve Monoprotic Or Polyprotic The way you normally carry out a titration involves adding the acid to the alkali. A summary of the important curves. The first marker is if the initial ph is above or below 7. Polyprotic acids are acids that can lose several protons per molecule. However, a polyprotic acid differs from a monoprotic acid because it has more than one. Titration Curve Monoprotic Or Polyprotic.
From www.youtube.com
Finding equivalence point for Monoprotic acid YouTube Titration Curve Monoprotic Or Polyprotic An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. A summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Therefore, it varies from 0. Titration Curve Monoprotic Or Polyprotic.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve Monoprotic Or Polyprotic Therefore, it varies from 0 to 1 for an acid that releases one proton. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Here are reduced versions of. The way you normally carry out a titration involves adding the acid to the alkali.. Titration Curve Monoprotic Or Polyprotic.
From www.grace.umd.edu
Simulation of Monoprotic Titration Curve Titration Curve Monoprotic Or Polyprotic However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Titrations of polyprotic acids have more than. The way you normally carry out a titration involves adding the acid to the alkali. The first marker is if the initial ph is above or below 7. Key features of titration curves: A. Titration Curve Monoprotic Or Polyprotic.
From www.bartleby.com
The graph shows the titration curves for two monoprotic Titration Curve Monoprotic Or Polyprotic However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. Polyprotic acids are acids that can lose several protons per molecule. The first marker is if the initial ph is above or below 7. Here are. Titration Curve Monoprotic Or Polyprotic.
From www.chegg.com
Solved Polyprotic Acids What can a titration curve tell us Titration Curve Monoprotic Or Polyprotic Polyprotic acids are acids that can lose several protons per molecule. Key features of titration curves: They can be further categorized into diprotic acids and triprotic acids, those which can donate two. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Titrations of polyprotic acids have more than. The first. Titration Curve Monoprotic Or Polyprotic.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Monoprotic Or Polyprotic Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Polyprotic acids are acids that can lose several protons per molecule. They can be further. Titration Curve Monoprotic Or Polyprotic.
From www.youtube.com
Titrations of Polyprotic Acids YouTube Titration Curve Monoprotic Or Polyprotic They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Titrations of polyprotic acids have more than. Key features of titration curves: The way you normally carry out a titration involves adding the acid to the alkali. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. Polyprotic acids. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
(1) Draw a generic pH meter titration curve for the titration of a Titration Curve Monoprotic Or Polyprotic An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Polyprotic acids are acids that can lose several protons per molecule. However, a polyprotic acid differs from a monoprotic. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
SOLVED The graph below shows the titration curves for two monoprotic Titration Curve Monoprotic Or Polyprotic They can be further categorized into diprotic acids and triprotic acids, those which can donate two. A summary of the important curves. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. The first marker is if the initial ph is above or below 7. Two key markers in a titration curve help us identify. Titration Curve Monoprotic Or Polyprotic.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Monoprotic Or Polyprotic Therefore, it varies from 0 to 1 for an acid that releases one proton. Titrations of polyprotic acids have more than. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. Here are reduced versions of. The first marker is if the initial ph is above or below 7. Polyprotic acids are acids that can. Titration Curve Monoprotic Or Polyprotic.
From www.transtutors.com
(Get Answer) The graph above shows the titration curves of four Titration Curve Monoprotic Or Polyprotic A summary of the important curves. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Polyprotic acids are acids that can lose several protons per molecule. Here are reduced versions of. Key features of titration curves: Titrations of polyprotic acids have more than. Two key markers in a titration curve. Titration Curve Monoprotic Or Polyprotic.
From www.youtube.com
Regions of Titration Curve and Polyprotic acids YouTube Titration Curve Monoprotic Or Polyprotic A summary of the important curves. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Titration Curve Monoprotic Or Polyprotic Titrations of polyprotic acids have more than. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. An arrhenius acid donates a proton (h+ h +), so a. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
SOLVED How does the titration curve of a monoprotic acid differ from Titration Curve Monoprotic Or Polyprotic They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Key features of titration curves: Titrations of polyprotic acids have more than. The way you normally carry out a titration involves adding the acid to the alkali. Therefore, it varies from 0 to 1 for an acid that releases one proton. Here are reduced. Titration Curve Monoprotic Or Polyprotic.
From www.savemyexams.com
Titration Curves of Polyprotic Acids College Board AP Chemistry Titration Curve Monoprotic Or Polyprotic Here are reduced versions of. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Polyprotic acids are acids that can lose several protons per molecule. The first marker is if the initial ph is above or below 7. A summary of the important. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
SOLVED Consider the overlaid titration curves for two monoprotic acids Titration Curve Monoprotic Or Polyprotic Here are reduced versions of. A summary of the important curves. Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Key features of titration curves: They can be further categorized into diprotic acids and triprotic acids, those which can donate two. However, a. Titration Curve Monoprotic Or Polyprotic.
From www.positivephysics.org
Acidbase Titrations Practice Questions Titrations Curves Polyprotic Titration Curve Monoprotic Or Polyprotic The way you normally carry out a titration involves adding the acid to the alkali. Key features of titration curves: However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. A summary of the important curves. An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons.. Titration Curve Monoprotic Or Polyprotic.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curve Monoprotic Or Polyprotic A summary of the important curves. Therefore, it varies from 0 to 1 for an acid that releases one proton. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Here are reduced versions of. The first marker is if the initial ph is above or below 7. An arrhenius acid. Titration Curve Monoprotic Or Polyprotic.
From forums.studentdoctor.net
Confusion about titration of polyprotic acids Student Doctor Network Titration Curve Monoprotic Or Polyprotic They can be further categorized into diprotic acids and triprotic acids, those which can donate two. Polyprotic acids are acids that can lose several protons per molecule. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. The first marker is if the initial ph is above or below 7. An. Titration Curve Monoprotic Or Polyprotic.
From brainly.com
The graphs labeled (a) and (b) show the titration curves for two equal Titration Curve Monoprotic Or Polyprotic An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. Here are reduced versions of. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Titrations of polyprotic acids have more than. The way you normally carry out a titration involves adding the acid to the. Titration Curve Monoprotic Or Polyprotic.
From www.studocu.com
Titration Curve and Polyprotic Acids WeakBase StrongAcid sHioNHtH3Ot Titration Curve Monoprotic Or Polyprotic An arrhenius acid donates a proton (h+ h +), so a polyprotic acid donates protons. Titrations of polyprotic acids have more than. Key features of titration curves: Two key markers in a titration curve help us identify whether the analyte and titrant in a titration is a strong or weak, acid or base. Here are reduced versions of. A summary. Titration Curve Monoprotic Or Polyprotic.
From www.chegg.com
Solved Look at the titration graphs below. Determine which Titration Curve Monoprotic Or Polyprotic Here are reduced versions of. Therefore, it varies from 0 to 1 for an acid that releases one proton. They can be further categorized into diprotic acids and triprotic acids, those which can donate two. The first marker is if the initial ph is above or below 7. Key features of titration curves: An arrhenius acid donates a proton (h+. Titration Curve Monoprotic Or Polyprotic.
From www.numerade.com
SOLVED ' The graph shows the titration curves for two Titration Curve Monoprotic Or Polyprotic Titrations of polyprotic acids have more than. The way you normally carry out a titration involves adding the acid to the alkali. A summary of the important curves. However, a polyprotic acid differs from a monoprotic acid because it has more than one acidic h+ h +,. Therefore, it varies from 0 to 1 for an acid that releases one. Titration Curve Monoprotic Or Polyprotic.
From www.slideserve.com
PPT Titration of Strong Monoprotic Acid and Base PowerPoint Titration Curve Monoprotic Or Polyprotic They can be further categorized into diprotic acids and triprotic acids, those which can donate two. The way you normally carry out a titration involves adding the acid to the alkali. Therefore, it varies from 0 to 1 for an acid that releases one proton. Polyprotic acids are acids that can lose several protons per molecule. Titrations of polyprotic acids. Titration Curve Monoprotic Or Polyprotic.