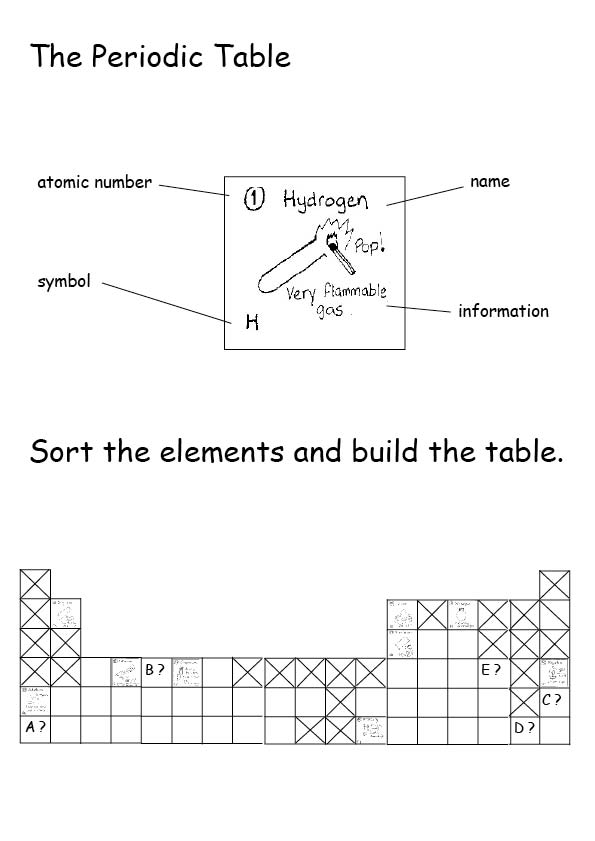
Chemistry Activity Table . Write an activity series for 3. the activity series is a chart of metals listed in order of declining relative reactivity. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Intro to general chemistry 3h 48m. define activity and determine which element in a reaction is more active given the balanced chemical equation. the activity series is a list of elements in decreasing order of their reactivity. he chart, that is it. be the first to ask a question about this topic. This means metals lose electrons more easily than nonmetals. Since metals replace other metals, while. If you do not have access to the chart you can assum. Metals are more easily oxidized than nonmetals. the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while.
from www.collaborativelearning.org
the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while. The top metals are more reactive than the metals on the bottom. Intro to general chemistry 3h 48m. he chart, that is it. be the first to ask a question about this topic. Write an activity series for 3. If you do not have access to the chart you can assum. the activity series is a list of elements in decreasing order of their reactivity. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq)
Science Chemistry activities
Chemistry Activity Table Intro to general chemistry 3h 48m. This means metals lose electrons more easily than nonmetals. Intro to general chemistry 3h 48m. Metals are more easily oxidized than nonmetals. the activity series is a chart of metals listed in order of declining relative reactivity. define activity and determine which element in a reaction is more active given the balanced chemical equation. Write an activity series for 3. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: The top metals are more reactive than the metals on the bottom. the activity series is a list of elements in decreasing order of their reactivity. Metals are strong reducing agents, but. be the first to ask a question about this topic. Since metals replace other metals, while. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while.
From www.pinterest.fr
Printable Periodic Tables (PDF) Chemistry classroom, Teaching Chemistry Activity Table Intro to general chemistry 3h 48m. Since metals replace other metals, while. Metals are more easily oxidized than nonmetals. be the first to ask a question about this topic. the activity series is a list of elements in decreasing order of their reactivity. This means metals lose electrons more easily than nonmetals. Since metals replace other metals, while.. Chemistry Activity Table.
From thestay-at-home-momsurvivalguide.com
Chemistry Activities for Kids MultiAge Learning Unit » The Stayat Chemistry Activity Table Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) the activity series is a list of elements in decreasing order of their reactivity. If you do not have access to the chart you can assum. Since metals replace other metals, while. For example, both magnesium and zinc can react with hydrogen ions to. Chemistry Activity Table.
From materialschoolpinder.z21.web.core.windows.net
Activity Series Definition Chemistry Chemistry Activity Table be the first to ask a question about this topic. Metals are more easily oxidized than nonmetals. the activity series is a list of elements in decreasing order of their reactivity. This means metals lose electrons more easily than nonmetals. Intro to general chemistry 3h 48m. Since metals replace other metals, while. Mg (s) + 2 h +. Chemistry Activity Table.
From familytipo.blogspot.com
Family and Parenting 50 Science Games for Kids that Make Learning Fun Chemistry Activity Table This means metals lose electrons more easily than nonmetals. be the first to ask a question about this topic. Since metals replace other metals, while. If you do not have access to the chart you can assum. the activity series is a chart of metals listed in order of declining relative reactivity. Write an activity series for 3.. Chemistry Activity Table.
From www.collaborativelearning.org
Science Chemistry activities Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. This means metals lose electrons more easily than nonmetals. Since metals replace other metals, while. be the first to ask a question about this topic. Since metals replace other metals, while. define activity and determine which element in a reaction is more active. Chemistry Activity Table.
From thestay-at-home-momsurvivalguide.com
freeprintablefillinworksheetforperiodictablechemistry Chemistry Activity Table Since metals replace other metals, while. If you do not have access to the chart you can assum. The top metals are more reactive than the metals on the bottom. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Since metals replace other metals, while. Intro to general chemistry 3h 48m. Metals are more. Chemistry Activity Table.
From materialschoolpinder.z21.web.core.windows.net
Activity Series List Chemistry Chemistry Activity Table the activity series is a chart of metals listed in order of declining relative reactivity. be the first to ask a question about this topic. If you do not have access to the chart you can assum. The top metals are more reactive than the metals on the bottom. This means metals lose electrons more easily than nonmetals.. Chemistry Activity Table.
From www.pinterest.co.uk
Homeschool Chemistry Handson Beginner's Guide To Atoms & The Periodic Chemistry Activity Table Metals are strong reducing agents, but. be the first to ask a question about this topic. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Metals are more easily oxidized than nonmetals. Intro to general chemistry 3h 48m. the activity series is a list of elements. Chemistry Activity Table.
From chem.libretexts.org
5.1 Activity Series Chemistry LibreTexts Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. define activity and determine which element in a reaction is more active given the balanced chemical equation. Metals are strong reducing agents, but. the activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while.. Chemistry Activity Table.
From www.artofit.org
21 fascinating periodic table activities for chemistry students of all Chemistry Activity Table he chart, that is it. This means metals lose electrons more easily than nonmetals. The top metals are more reactive than the metals on the bottom. define activity and determine which element in a reaction is more active given the balanced chemical equation. For example, both magnesium and zinc can react with hydrogen ions to displace h 2. Chemistry Activity Table.
From homeschoolden.com
Chemistry Activities The First 20 Elements of the Periodic Table Chemistry Activity Table Since metals replace other metals, while. Since metals replace other metals, while. he chart, that is it. Intro to general chemistry 3h 48m. The top metals are more reactive than the metals on the bottom. Metals are strong reducing agents, but. If you do not have access to the chart you can assum. This means metals lose electrons more. Chemistry Activity Table.
From printableschoolschulths.z19.web.core.windows.net
Chemistry Periodic Table Worksheet Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. Metals are more easily oxidized than nonmetals. the activity series is a list of elements in decreasing order of their reactivity. he chart, that is it. be the first to ask a question about this topic. the activity series is a. Chemistry Activity Table.
From old.sermitsiaq.ag
Chemistry Printable Periodic Table Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. Intro to general chemistry 3h 48m. the activity series is a list of elements in decreasing order of their reactivity. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: he chart,. Chemistry Activity Table.
From www.pinterest.cl
The History of the Periodic Table of Elements Grab your free science Chemistry Activity Table If you do not have access to the chart you can assum. define activity and determine which element in a reaction is more active given the balanced chemical equation. Since metals replace other metals, while. be the first to ask a question about this topic. Mg (s) + 2 h + (aq) → h 2 (g) + mg. Chemistry Activity Table.
From printableyamertua.z19.web.core.windows.net
Periodic Table Worksheet Chemistry Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. Metals are strong reducing agents, but. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Since metals replace other metals, while. define activity and determine which element in a reaction is more active given the balanced chemical equation.. Chemistry Activity Table.
From materialschoolpinder.z21.web.core.windows.net
Activity Series Chemistry Chart Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. This means metals lose electrons more easily than nonmetals. The top metals are more reactive than the metals on the bottom. be the first to ask a question about this topic. define activity and determine which element in a reaction is more active. Chemistry Activity Table.
From www.pinterest.es
FREE Printable Make Your Own Periodic Table Worksheets Activity Matter Chemistry Activity Table he chart, that is it. This means metals lose electrons more easily than nonmetals. Since metals replace other metals, while. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Intro to general chemistry 3h 48m. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution. Chemistry Activity Table.
From sciencenotes.org
Periodic Table Worksheets Chemistry Activity Table Metals are more easily oxidized than nonmetals. the activity series is a chart of metals listed in order of declining relative reactivity. The top metals are more reactive than the metals on the bottom. If you do not have access to the chart you can assum. the activity series is a list of elements in decreasing order of. Chemistry Activity Table.
From www.pinterest.co.uk
Have fun and learn about the periodic table with this fun elemental Chemistry Activity Table Since metals replace other metals, while. the activity series is a chart of metals listed in order of declining relative reactivity. Intro to general chemistry 3h 48m. If you do not have access to the chart you can assum. The top metals are more reactive than the metals on the bottom. be the first to ask a question. Chemistry Activity Table.
From thestay-at-home-momsurvivalguide.com
periodic table matching game chemistry activities for kids The Stay Chemistry Activity Table This means metals lose electrons more easily than nonmetals. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Write an activity series for 3. Since metals replace other metals, while. he chart, that is it. Since metals replace other metals, while. Intro to general chemistry 3h 48m. the activity series is a. Chemistry Activity Table.
From thestay-at-home-momsurvivalguide.com
Chemistry Activities for Kids MultiAge Learning Unit » The Stayat Chemistry Activity Table be the first to ask a question about this topic. the activity series is a chart of metals listed in order of declining relative reactivity. If you do not have access to the chart you can assum. Since metals replace other metals, while. the activity series is a list of elements in decreasing order of their reactivity.. Chemistry Activity Table.
From sl-713.weebly.com
Chemistry Reference SL713 Chemistry Activity Table the activity series is a chart of metals listed in order of declining relative reactivity. he chart, that is it. Write an activity series for 3. Since metals replace other metals, while. This means metals lose electrons more easily than nonmetals. Metals are more easily oxidized than nonmetals. Intro to general chemistry 3h 48m. Metals are strong reducing. Chemistry Activity Table.
From thestay-at-home-momsurvivalguide.com
usingtheperiodictabletofillininfochemistryactivitiesforkids Chemistry Activity Table This means metals lose electrons more easily than nonmetals. define activity and determine which element in a reaction is more active given the balanced chemical equation. the activity series is a list of elements in decreasing order of their reactivity. The top metals are more reactive than the metals on the bottom. Metals are more easily oxidized than. Chemistry Activity Table.
From mungfali.com
Chemistry Periodic Table Printable Chemistry Activity Table Since metals replace other metals, while. Metals are strong reducing agents, but. Write an activity series for 3. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: the activity series is a chart of metals listed in order of declining relative reactivity. he chart, that is. Chemistry Activity Table.
From studylibraryhaiques.z21.web.core.windows.net
Periodic Table Activities For Kids Chemistry Activity Table If you do not have access to the chart you can assum. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Write an activity series for 3. be the first to ask a question about this topic. Metals are more easily oxidized than nonmetals. Metals are strong. Chemistry Activity Table.
From www.pinterest.com
This is an awesome engagement activity for balancing chemical equations Chemistry Activity Table Metals are more easily oxidized than nonmetals. the activity series is a list of elements in decreasing order of their reactivity. If you do not have access to the chart you can assum. Write an activity series for 3. the activity series is a list of elements in decreasing order of their reactivity. For example, both magnesium and. Chemistry Activity Table.
From www.pinterest.com
Periodic Table Activity Worksheets Interactive Periodic Table Chemistry Activity Table the activity series is a chart of metals listed in order of declining relative reactivity. Metals are more easily oxidized than nonmetals. Since metals replace other metals, while. he chart, that is it. be the first to ask a question about this topic. Intro to general chemistry 3h 48m. Since metals replace other metals, while. the. Chemistry Activity Table.
From www.youtube.com
Activity Series, SAT Chemistry Review 8 YouTube Chemistry Activity Table This means metals lose electrons more easily than nonmetals. Since metals replace other metals, while. Metals are more easily oxidized than nonmetals. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: The top metals are more reactive than the metals on the bottom. Metals are strong reducing agents,. Chemistry Activity Table.
From www.pinterest.com
Periodic Table of Elements Activity Construct a Simple One—A Visual Chemistry Activity Table be the first to ask a question about this topic. define activity and determine which element in a reaction is more active given the balanced chemical equation. he chart, that is it. If you do not have access to the chart you can assum. Write an activity series for 3. the activity series is a chart. Chemistry Activity Table.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Chemistry Activity Table Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) The top metals are more reactive than the metals on the bottom. be the first to ask a question about this topic. define activity and determine which element in a reaction is more active given the balanced chemical equation. Since metals replace other. Chemistry Activity Table.
From ame.my.id
Periodic Table Activity Worksheet Ame.my.id Chemistry Activity Table Intro to general chemistry 3h 48m. If you do not have access to the chart you can assum. Since metals replace other metals, while. the activity series is a list of elements in decreasing order of their reactivity. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions:. Chemistry Activity Table.
From infoupdate.org
Periodic Table Activity Series Of Metals Chemistry Activity Table Since metals replace other metals, while. Write an activity series for 3. This means metals lose electrons more easily than nonmetals. define activity and determine which element in a reaction is more active given the balanced chemical equation. Intro to general chemistry 3h 48m. Metals are more easily oxidized than nonmetals. For example, both magnesium and zinc can react. Chemistry Activity Table.
From www.pinterest.com
Activity Series can be used to predict the of reactions Chemistry Activity Table define activity and determine which element in a reaction is more active given the balanced chemical equation. he chart, that is it. For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: If you do not have access to the chart you can assum. This means metals. Chemistry Activity Table.
From www.pinterest.com
Periodic Table of Elements ActivityAtomic Structure and the Periodic Chemistry Activity Table the activity series is a list of elements in decreasing order of their reactivity. The top metals are more reactive than the metals on the bottom. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) This means metals lose electrons more easily than nonmetals. the activity series is a list of elements. Chemistry Activity Table.
From www.pinterest.com.au
A fun activity for revising elements of the periodic table and Chemistry Activity Table This means metals lose electrons more easily than nonmetals. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Write an activity series for 3. Metals are strong reducing agents, but. If you do. Chemistry Activity Table.