Catalyst Experiment Explanation . Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases the. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. It is not consumed by the process. Autocatalyst = a reaction product which is a.
from smartcatalyst.ir
Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. A catalyst lowers the activation energy of a reaction, increasing its rate. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. In chemistry and biology, a catalyst is a substance the increases the. Autocatalyst = a reaction product which is a. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself.
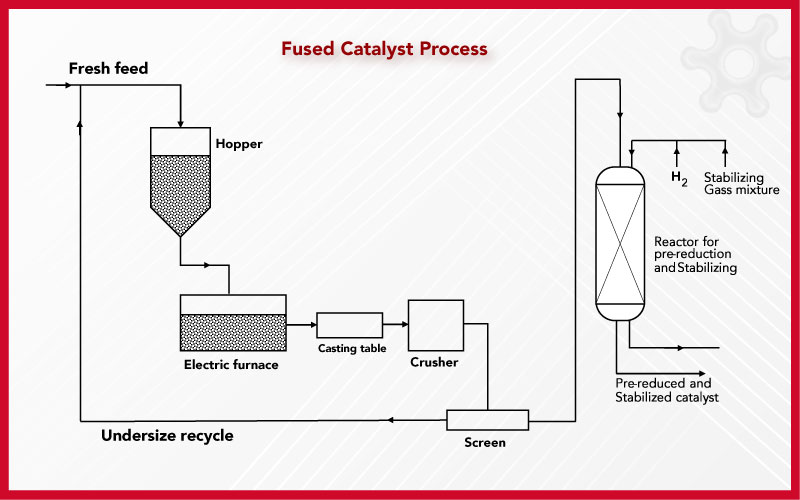
Various Methods Of Preparing Iron Catalysts
Catalyst Experiment Explanation A catalyst lowers the activation energy of a reaction, increasing its rate. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. In chemistry and biology, a catalyst is a substance the increases the. It is not consumed by the process. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. A catalyst lowers the activation energy of a reaction, increasing its rate. Autocatalyst = a reaction product which is a. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Catalyst Experiment Explanation An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. From fresh liver, to powdered manganese, create different catalysts. Catalyst Experiment Explanation.
From owlcation.com
Chemical Reactions and Chemical Equations Owlcation Catalyst Experiment Explanation An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. It is not consumed by the process. From fresh liver, to powdered. Catalyst Experiment Explanation.
From www.slideshare.net
Biology 2.4 Catalyst Experiment Explanation Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Autocatalyst = a reaction product which is a. A catalyst lowers the activation energy of a reaction, increasing its rate. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. In this catalase and hydrogen peroxide experiment, we will. Catalyst Experiment Explanation.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Experiment Explanation An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. In chemistry and biology, a catalyst is a substance the increases the. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. A catalyst lowers the activation energy of a reaction, increasing its rate. Autocatalyst = a reaction product which. Catalyst Experiment Explanation.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Experiment Explanation It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. A. Catalyst Experiment Explanation.
From www.tes.com
Catalysts lesson and experiment Teaching Resources Catalyst Experiment Explanation Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. Autocatalyst = a reaction product which is a. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. An. Catalyst Experiment Explanation.
From klamsflsd.blob.core.windows.net
Catalyst Change A Reaction at Rebecca Miller blog Catalyst Experiment Explanation Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. In chemistry and biology, a catalyst is a substance the increases the. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. It is not consumed by the process. Autocatalyst = a reaction product which is a. An entity. Catalyst Experiment Explanation.
From exoieyaef.blob.core.windows.net
Catalyst Def Biology at Ethel Hughes blog Catalyst Experiment Explanation It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. In this catalase and hydrogen peroxide experiment, we. Catalyst Experiment Explanation.
From www.researchgate.net
6 Schematic diagram of hydrothermal method of synthesis. Download Catalyst Experiment Explanation Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Autocatalyst = a reaction product which is a. To deal with this,. Catalyst Experiment Explanation.
From klayfvveg.blob.core.windows.net
Role Of A Catalyst In The Human Body at Jerald Fournier blog Catalyst Experiment Explanation In chemistry and biology, a catalyst is a substance the increases the. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. In this. Catalyst Experiment Explanation.
From edu.rsc.org
Cracking hydrocarbons in liquid paraffin with a catalyst Experiment Catalyst Experiment Explanation It is not consumed by the process. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. Catalyst precursor = a compound or complex which undergoes a reaction to produce. Catalyst Experiment Explanation.
From smartcatalyst.ir
Various Methods Of Preparing Iron Catalysts Catalyst Experiment Explanation A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases the. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. It is not consumed by the process. From fresh liver, to powdered manganese, create. Catalyst Experiment Explanation.
From www.youtube.com
Demonstration of a Simple Catalyst Experiment YouTube Catalyst Experiment Explanation A catalyst lowers the activation energy of a reaction, increasing its rate. It is not consumed by the process. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. In this catalase and hydrogen peroxide. Catalyst Experiment Explanation.
From theory.cm.utexas.edu
1. R. M. Anderson, D. F. Yancey, L. Zhang, S. T. Chill, G. Henkelman Catalyst Experiment Explanation It is not consumed by the process. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a. Catalyst Experiment Explanation.
From www.mdpi.com
Catalysts Free FullText New Method of Determining Catalyst Experiment Explanation An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. Autocatalyst = a reaction product which is a. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions. Catalyst Experiment Explanation.
From borates.today
Catalytic Cracking Processes With Boron Catalyst Borates Today Catalyst Experiment Explanation From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. An entity (organic, inorganic, organometallic, protein or. Catalyst Experiment Explanation.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Experiment Explanation To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent. Catalyst Experiment Explanation.
From joiughueo.blob.core.windows.net
How To Get Rid Of Oxygen From Water at Joe Thompson blog Catalyst Experiment Explanation It is not consumed by the process. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. In chemistry and biology, a catalyst is a substance the increases the. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. Autocatalyst =. Catalyst Experiment Explanation.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Catalyst Experiment Explanation Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. In chemistry and biology, a catalyst is a substance the increases the. An. Catalyst Experiment Explanation.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Experiment Explanation To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. In chemistry and biology, a catalyst is a substance the increases the. Catalysts affect the rate of a chemical reaction. Catalyst Experiment Explanation.
From slideplayer.com
Electric field emission and local surface heating in plasma packed bed Catalyst Experiment Explanation Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. Autocatalyst = a reaction. Catalyst Experiment Explanation.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalyst Experiment Explanation Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Catalysts affect the rate of. Catalyst Experiment Explanation.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalyst Experiment Explanation In chemistry and biology, a catalyst is a substance the increases the. It is not consumed by the process. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Autocatalyst = a. Catalyst Experiment Explanation.
From exocpyweq.blob.core.windows.net
Catalyst Explanation at Sandra Nagle blog Catalyst Experiment Explanation Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. It is not consumed by the process. In chemistry and biology, a catalyst is. Catalyst Experiment Explanation.
From joicdqxxo.blob.core.windows.net
Catalyst Development Definition at Ruth Cruz blog Catalyst Experiment Explanation To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. Autocatalyst = a reaction product which is a. It is not consumed by the process. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst lowers the activation. Catalyst Experiment Explanation.
From www.mdpi.com
Catalysts Free FullText Biodiesel Production Using Solid Acid Catalyst Experiment Explanation Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. A catalyst lowers the activation energy of a reaction, increasing its rate. It is not consumed by the process. In chemistry and biology, a catalyst is a. Catalyst Experiment Explanation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Experiment Explanation An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalyst precursor = a compound or complex which undergoes a reaction to. Catalyst Experiment Explanation.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Experiment Explanation In chemistry and biology, a catalyst is a substance the increases the. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. Catalyst precursor = a compound or complex which undergoes. Catalyst Experiment Explanation.
From exocpyweq.blob.core.windows.net
Catalyst Explanation at Sandra Nagle blog Catalyst Experiment Explanation Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. An entity (organic, inorganic, organometallic, protein or rna) that increases. Catalyst Experiment Explanation.
From joilemviq.blob.core.windows.net
Catalyst In Reaction Purpose at John Gainey blog Catalyst Experiment Explanation From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical reactions to occur. An entity (organic,. Catalyst Experiment Explanation.
From www.nagwa.com
Question Video Identifying the Correct Statement For the Catalyst Experiment Explanation To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. In chemistry and biology, a catalyst is a substance the increases the. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. From fresh liver, to powdered manganese, create different catalysts. Catalyst Experiment Explanation.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Catalyst Experiment Explanation A catalyst lowers the activation energy of a reaction, increasing its rate. Autocatalyst = a reaction product which is a. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. In. Catalyst Experiment Explanation.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Experiment Explanation A catalyst lowers the activation energy of a reaction, increasing its rate. Catalyst precursor = a compound or complex which undergoes a reaction to produce the active catalyst. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Autocatalyst = a reaction product which is a. From fresh liver, to powdered manganese,. Catalyst Experiment Explanation.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Catalyst Experiment Explanation From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide decomposition. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous. To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. Catalyst precursor = a compound or complex. Catalyst Experiment Explanation.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Experiment Explanation To deal with this, living organisms produce an enzyme (a catalyst) that breaks down hydrogen peroxide before it can do much damage. It is not consumed by the process. An entity (organic, inorganic, organometallic, protein or rna) that increases the rate of a reaction without itself. A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry. Catalyst Experiment Explanation.