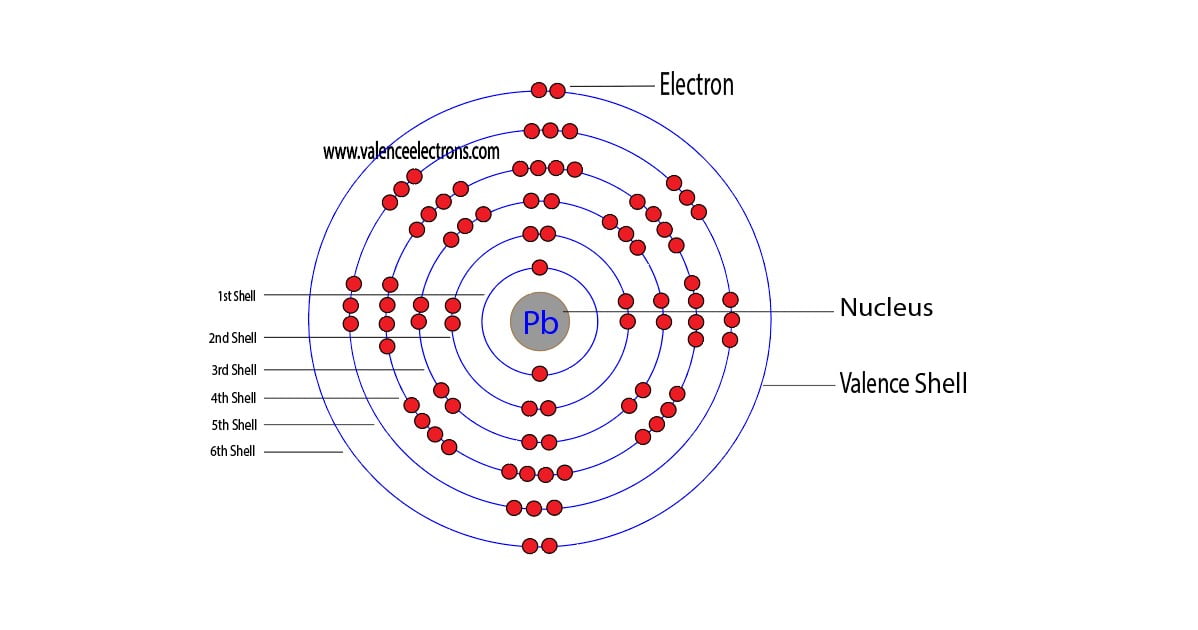
Lead Orbital Electrons . The sum of lead's first and second ionization energies—the total energy required to remove. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The atomic number of lead represents the total number of electrons of lead. This electron configuration shows that the lead ion (pb 4+). Since the atomic number of lead is 82, the total electrons. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The ground state electronic configuration of neutral lead is [xe]. The shorthand electron configuration for lead is: Learn the basics of electron configurations before attempting to write out. [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. Lead electron configuration | image: 6p2 and the term symbol of lead is.
from valenceelectrons.com
6p2 and the term symbol of lead is. The atomic number of lead represents the total number of electrons of lead. [xe] 6s 2 4f 14 5d 10 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6. This electron configuration shows that the lead ion (pb 4+). Learn the basics of electron configurations before attempting to write out. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. The shorthand electron configuration for lead is: Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Lead electron configuration | image:
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+)
Lead Orbital Electrons Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Lead electron configuration | image: This electron configuration shows that the lead ion (pb 4+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6. 6p2 and the term symbol of lead is. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Since the atomic number of lead is 82, the total electrons. The ground state electronic configuration of neutral lead is [xe]. [xe] 6s 2 4f 14 5d 10 6p 2. Learn the basics of electron configurations before attempting to write out. The sum of lead's first and second ionization energies—the total energy required to remove. The atomic number of lead represents the total number of electrons of lead. The shorthand electron configuration for lead is: A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2.
From www.reddit.com
What is the path an electron follows around the nucleus? r/askscience Lead Orbital Electrons A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. This electron configuration shows that the lead ion (pb 4+). [xe] 6s 2 4f 14 5d 10 6p 2. The atomic number of lead represents the total number of electrons of lead. 6p2 and the term symbol of lead is.. Lead Orbital Electrons.
From circuitlibthrusts.z13.web.core.windows.net
Lead Orbital Diagram Lead Orbital Electrons The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Learn the basics of electron configurations. Lead Orbital Electrons.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica Lead Orbital Electrons 6p2 and the term symbol of lead is. Lead electron configuration | image: This electron configuration shows that the lead ion (pb 4+). The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p. Lead Orbital Electrons.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Orbital Electrons This electron configuration shows that the lead ion (pb 4+). The sum of lead's first and second ionization energies—the total energy required to remove. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Since. Lead Orbital Electrons.
From courses.lumenlearning.com
Electrons Biology for Majors I Lead Orbital Electrons Lead electron configuration | image: A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. Since the atomic number of lead is 82, the total electrons. The atomic number of lead represents the total number of electrons of lead. [xe] 6s 2 4f 14 5d 10 6p 2. The electron. Lead Orbital Electrons.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead Orbital Electrons 6p2 and the term symbol of lead is. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. [xe] 6s 2 4f 14 5d 10 6p 2. Lead electron configuration | image: The atomic number of lead represents the total number of electrons of lead. This electron configuration shows that the lead ion (pb 4+). The shorthand electron configuration. Lead Orbital Electrons.
From ar.inspiredpencil.com
Electron Configuration Diagram Orbitals Lead Orbital Electrons Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Learn the basics of electron configurations before attempting to write out. This electron configuration shows that the lead ion (pb 4+). The atomic number of lead represents the total number. Lead Orbital Electrons.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead Orbital Electrons 6p2 and the term symbol of lead is. The ground state electronic configuration of neutral lead is [xe]. [xe] 6s 2 4f 14 5d 10 6p 2. The atomic number of lead represents the total number of electrons of lead. Learn the basics of electron configurations before attempting to write out. The lead electron configuration, represented as [xe] 6s 2. Lead Orbital Electrons.
From borislayson.blogspot.com
iodine orbital diagram BorisLayson Lead Orbital Electrons The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. [xe] 6s 2 4f 14 5d 10 6p 2. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10. Lead Orbital Electrons.
From www.youtube.com
CHEMISTRY 101 Valence and core electrons YouTube Lead Orbital Electrons A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. Learn the basics of electron configurations before attempting to write out. Lead electron configuration | image: The ground state electronic configuration of neutral lead is [xe]. The shorthand electron configuration for lead is: This electron configuration shows that the lead. Lead Orbital Electrons.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Orbital Electrons The sum of lead's first and second ionization energies—the total energy required to remove. The atomic number of lead represents the total number of electrons of lead. The shorthand electron configuration for lead is: Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2. Lead Orbital Electrons.
From valenceelectrons.com
Electron Configuration for Lead and Lead ions(Pb2+, Pb4+) Lead Orbital Electrons Since the atomic number of lead is 82, the total electrons. [xe] 6s 2 4f 14 5d 10 6p 2. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The. Lead Orbital Electrons.
From saylordotorg.github.io
Quantum Numbers for Electrons Lead Orbital Electrons Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Learn the basics of electron configurations before attempting to write out. The ground state electronic configuration of neutral lead is [xe]. 6p2 and the term symbol of lead is. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2.. Lead Orbital Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Orbital Electrons The shorthand electron configuration for lead is: The ground state electronic configuration of neutral lead is [xe]. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Learn the basics of electron configurations before attempting to write out. [xe] 6s 2 4f 14 5d 10 6p 2. The electron configuration of lead (pb) is described by its atomic structure,. Lead Orbital Electrons.
From pixels.com
Bohr Transitions For Electron Orbitals Photograph by Science Photo Library Lead Orbital Electrons Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. [xe] 6s 2 4f 14 5d 10 6p 2. This electron configuration shows that the lead ion (pb 4+). Since the atomic number of lead is 82, the total electrons. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s. Lead Orbital Electrons.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Lead Orbital Electrons The ground state electronic configuration of neutral lead is [xe]. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. [xe] 6s 2 4f 14 5d 10 6p 2. The atomic number of lead represents the total number of electrons of lead. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2. Lead Orbital Electrons.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica Lead Orbital Electrons [xe] 6s 2 4f 14 5d 10 6p 2. The shorthand electron configuration for lead is: The atomic number of lead represents the total number of electrons of lead. The sum of lead's first and second ionization energies—the total energy required to remove. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2. Lead Orbital Electrons.
From gzscienceclassonline.weebly.com
1. Electron Configuration Lead Orbital Electrons The ground state electronic configuration of neutral lead is [xe]. Learn the basics of electron configurations before attempting to write out. The sum of lead's first and second ionization energies—the total energy required to remove. The shorthand electron configuration for lead is: [xe] 6s 2 4f 14 5d 10 6p 2. A lead atom has 82 electrons, arranged in an. Lead Orbital Electrons.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Orbital Electrons The shorthand electron configuration for lead is: The sum of lead's first and second ionization energies—the total energy required to remove. This electron configuration shows that the lead ion (pb 4+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2. Lead Orbital Electrons.
From dreamstime.com
Diagram Representation Of The Element Lead Stock Vector Image 59012885 Lead Orbital Electrons The shorthand electron configuration for lead is: The sum of lead's first and second ionization energies—the total energy required to remove. Learn the basics of electron configurations before attempting to write out. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. A lead atom has 82 electrons, arranged in an. Lead Orbital Electrons.
From mavink.com
Electron Configuration Chart With Orbitals Lead Orbital Electrons The sum of lead's first and second ionization energies—the total energy required to remove. The shorthand electron configuration for lead is: Learn the basics of electron configurations before attempting to write out. The ground state electronic configuration of neutral lead is [xe]. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2. Lead Orbital Electrons.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Orbital Electrons Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The atomic number of lead represents the total number of electrons of lead. Lead. Lead Orbital Electrons.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Lead Orbital Electrons The shorthand electron configuration for lead is: Since the atomic number of lead is 82, the total electrons. Learn the basics of electron configurations before attempting to write out. This electron configuration shows that the lead ion (pb 4+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The atomic number of lead represents the total number of. Lead Orbital Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Lead Orbital Electrons The sum of lead's first and second ionization energies—the total energy required to remove. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The shorthand electron configuration for lead is: Lead electron configuration | image: The atomic number of. Lead Orbital Electrons.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Lead Orbital Electrons The ground state electronic configuration of neutral lead is [xe]. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The shorthand electron configuration for lead is: The lead electron configuration, represented as [xe] 6s. Lead Orbital Electrons.
From www.researchgate.net
Illustration of the orbital interactions that lead to lone pair Lead Orbital Electrons Since the atomic number of lead is 82, the total electrons. This electron configuration shows that the lead ion (pb 4+). Lead electron configuration | image: The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The ground state electronic configuration of neutral lead is [xe]. The sum of lead's first. Lead Orbital Electrons.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Lead Orbital Electrons The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The atomic number of lead represents the total number of electrons of lead. [xe] 6s 2 4f 14 5d 10 6p 2. A lead atom. Lead Orbital Electrons.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table Lead Orbital Electrons 6p2 and the term symbol of lead is. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Since the atomic number of lead is 82, the total electrons. [xe] 6s 2 4f 14 5d. Lead Orbital Electrons.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Orbital Electrons This electron configuration shows that the lead ion (pb 4+). Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s. Lead Orbital Electrons.
From wiringfixunripping.z21.web.core.windows.net
Valence Electrons Orbital Diagram Lead Orbital Electrons The atomic number of lead represents the total number of electrons of lead. Since the atomic number of lead is 82, the total electrons. 6p2 and the term symbol of lead is. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Lead Orbital Electrons.
From www.alamy.com
Lead (Pb). Diagram of the nuclear composition, electron configuration Lead Orbital Electrons The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6. The sum of lead's first and second ionization energies—the total energy required to remove. A lead atom has 82 electrons, arranged in. Lead Orbital Electrons.
From www.expii.com
Orbital Diagrams — Overview & Examples Expii Lead Orbital Electrons The atomic number of lead represents the total number of electrons of lead. The ground state electronic configuration of neutral lead is [xe]. 6p2 and the term symbol of lead is. Lead electron configuration | image: The sum of lead's first and second ionization energies—the total energy required to remove. Learn the basics of electron configurations before attempting to write. Lead Orbital Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Orbital Electrons The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s. Lead Orbital Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Orbital Electrons [xe] 6s 2 4f 14 5d 10 6p 2. The sum of lead's first and second ionization energies—the total energy required to remove. 6p2 and the term symbol of lead is. The atomic number of lead represents the total number of electrons of lead. The ground state electronic configuration of neutral lead is [xe]. The electron configuration of lead ion. Lead Orbital Electrons.
From chem.libretexts.org
8.5 Molecular Orbital Theory Chemistry LibreTexts Lead Orbital Electrons A lead atom has 82 electrons, arranged in an electron configuration of 4f 14 5d 10 6s 2 6p 2. Since the atomic number of lead is 82, the total electrons. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The sum of lead's first and second ionization energies—the total. Lead Orbital Electrons.