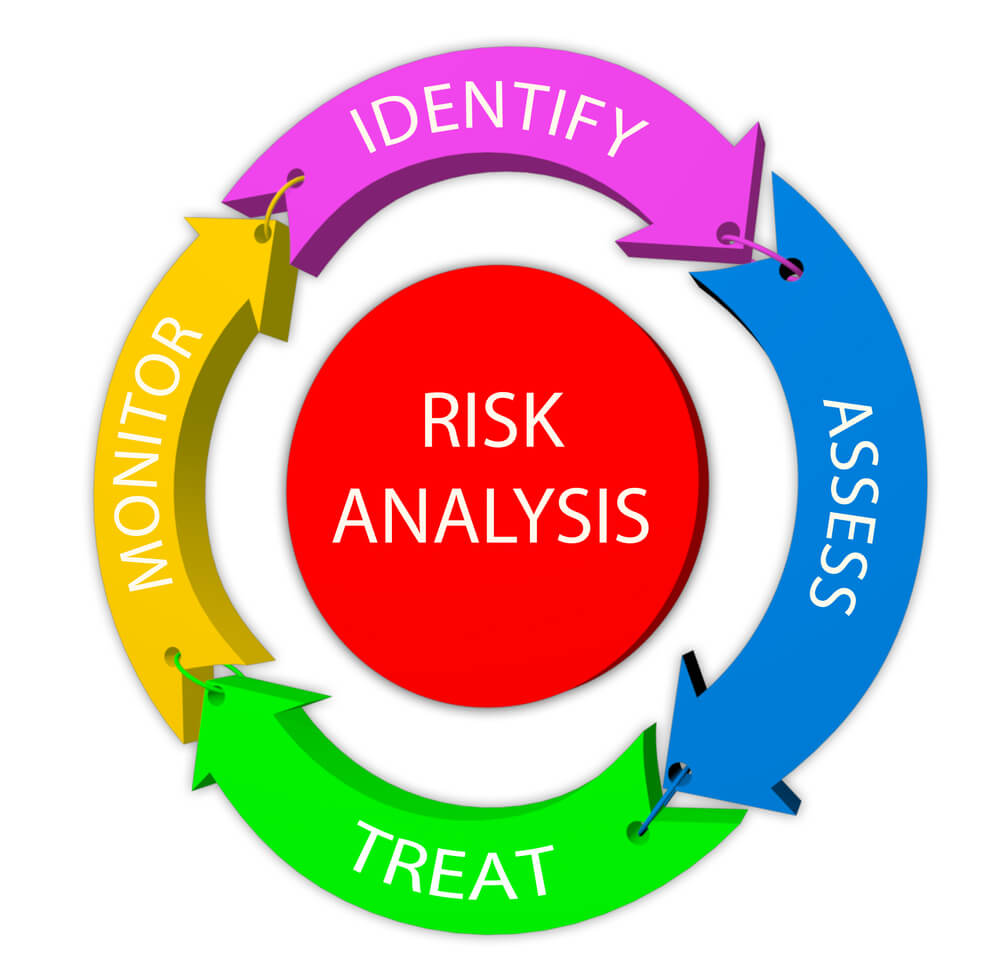
What Is Risk Assessment In Pharma . Develop a robust quantitative view of which risks matter most. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. This document provides principles and examples of tools for quality risk management that can be applied to different. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. An overall and continuing systematic process for the assessment, control, communication and. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar.
from www.oneeducation.org.uk
Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. An overall and continuing systematic process for the assessment, control, communication and. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Develop a robust quantitative view of which risks matter most. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. This document provides principles and examples of tools for quality risk management that can be applied to different. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as.
Dynamic Risk Assessment Why Do You Need This? Download Template
What Is Risk Assessment In Pharma Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. Develop a robust quantitative view of which risks matter most. This document provides principles and examples of tools for quality risk management that can be applied to different. An overall and continuing systematic process for the assessment, control, communication and. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for.
From www.medsnews.com
5 Risk Management Steps In The Pharmaceutical Industry What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. 2.1 the holder of. What Is Risk Assessment In Pharma.
From www.qbdvision.com
Focus on FMEA Process Risk Assessments (Part 3) QbDVision What Is Risk Assessment In Pharma 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Effective. What Is Risk Assessment In Pharma.
From www.lucidchart.com
A Complete Guide to the Risk Assessment Process Lucidchart Blog What Is Risk Assessment In Pharma Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. This document provides principles and examples of tools for quality risk management that can be applied to different.. What Is Risk Assessment In Pharma.
From www.semanticscholar.org
Critical Risk Assessment and Management in Pharmaceutical Industry What Is Risk Assessment In Pharma Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Develop a robust quantitative view of which risks matter most. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they. What Is Risk Assessment In Pharma.
From pharmabeginers.com
SOP for Quality Risk Management (Guideline ICH Q9) Pharma Beginners What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. An overall and continuing systematic process for the assessment, control, communication and. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as.. What Is Risk Assessment In Pharma.
From www.azonano.com
Risk Assessment in Pharmaceutical Production What Is Risk Assessment In Pharma Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Develop a robust quantitative view of which risks matter most. Risk assessment, as it is described in ich q9, is a systematic. What Is Risk Assessment In Pharma.
From qbdworks.com
Risk Assessment Process What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part. What Is Risk Assessment In Pharma.
From powerslides.com
Risk Assessment Template Download Now PowerSlides™ What Is Risk Assessment In Pharma Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. This document provides principles and examples of tools for quality risk management that can be applied to different. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Effective risk management begins with. What Is Risk Assessment In Pharma.
From pharmadevils.com
Quality Risk Assessment in Pharma What Is Risk Assessment In Pharma Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. An. What Is Risk Assessment In Pharma.
From www.pharmaceuticalonline.com
Introduction To The ASTM E3106 “Standard Guide To ScienceBased And What Is Risk Assessment In Pharma An overall and continuing systematic process for the assessment, control, communication and. Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are. What Is Risk Assessment In Pharma.
From www.sampletemplatess.com
Pharmaceutical Risk Assessment Template SampleTemplatess What Is Risk Assessment In Pharma 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. Develop a robust quantitative view of which risks matter most.. What Is Risk Assessment In Pharma.
From www.semanticscholar.org
[PDF] A Risk Modeling Framework for the Pharmaceutical Industry What Is Risk Assessment In Pharma Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. This document provides principles and examples of tools for quality risk management that can be applied to different. An overall and continuing systematic process for the assessment, control, communication and. Risk assessment consists of the identification of hazards and the analysis and evaluation. What Is Risk Assessment In Pharma.
From www.oneeducation.org.uk
Dynamic Risk Assessment Why Do You Need This? Download Template What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. An overall and continuing systematic process for the assessment, control, communication and. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are. What Is Risk Assessment In Pharma.
From tech-publish.com
Nitrosamine Impurities Quality Risk Management 2022 Techpublish What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. An overall and continuing systematic process for the. What Is Risk Assessment In Pharma.
From www.sampletemplatess.com
Manufacturing Risk Assessment Template SampleTemplatess What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. Acquire sufficient product and process knowledge to assess risks associated with formulation. What Is Risk Assessment In Pharma.
From www.riskpal.com
Risk Assessment Matrices Tools to Visualise Risk What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify,. What Is Risk Assessment In Pharma.
From clinlabworld.blogspot.com
clinical laboratory RISK MANAGEMENT IN CLINICAL LABORATOR What Is Risk Assessment In Pharma An overall and continuing systematic process for the assessment, control, communication and. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. This document. What Is Risk Assessment In Pharma.
From www.researchgate.net
Key considerations in the assessment of risk from biopharmaceutics What Is Risk Assessment In Pharma 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. An overall and continuing systematic process for the assessment, control, communication and. Develop a robust quantitative view of which risks matter most. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Risk assessment. What Is Risk Assessment In Pharma.
From www.johnandrewsrm.com
Risk Assessments what they are, why they're important and how to What Is Risk Assessment In Pharma 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Develop a robust quantitative view of which risks matter most. This document provides principles and examples of tools for quality risk management that can be applied to different. Acquire sufficient product and process knowledge to assess risks associated with formulation. What Is Risk Assessment In Pharma.
From www.linkedin.com
Step wise approach for the Quality Risk Management (QRM) in What Is Risk Assessment In Pharma Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. This document provides principles and examples of tools for quality risk management that can be applied to different. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for.. What Is Risk Assessment In Pharma.
From www.kevinian.com
How to Complete a Risk Assessment Kevin Ian Schmidt What Is Risk Assessment In Pharma Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. Risk assessment consists of the identification of hazards and the analysis and evaluation. What Is Risk Assessment In Pharma.
From www.businesswire.com
Pharmaceutical Packaging Industry Risk Assessment A Case Study on What Is Risk Assessment In Pharma Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. 2.1 the holder of a. What Is Risk Assessment In Pharma.
From www.americanpharmaceuticalreview.com
Critical Quality Attributes Assessment and Testing Strategy for What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. An overall and continuing systematic process for the assessment, control, communication and.. What Is Risk Assessment In Pharma.
From www.vrogue.co
Risk Assessment In Pharmaceutical Industry vrogue.co What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part. What Is Risk Assessment In Pharma.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Develop a robust quantitative view of which risks matter most. An overall and continuing systematic process for the assessment, control, communication and.. What Is Risk Assessment In Pharma.
From www.businessanalyststoolkit.com
How to Use Solution Assessment Criteria in a Business Case What Is Risk Assessment In Pharma 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. An overall and continuing systematic process for the assessment, control, communication and. This document provides principles and examples of tools for quality risk. What Is Risk Assessment In Pharma.
From mungfali.com
Risk Assessment Logo What Is Risk Assessment In Pharma Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. This document provides principles and examples of tools for quality risk management that can be applied to different. Acquire sufficient product and. What Is Risk Assessment In Pharma.
From www.scribd.com
Risk Management Plan Pharmacy Prescription Drugs What Is Risk Assessment In Pharma Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. This document provides principles and examples of tools for quality risk management that can be applied to different. Effective risk management begins with a robust process to identify, quantify, and. What Is Risk Assessment In Pharma.
From www.slideteam.net
Risk Assessment Of Critical Quality Attributes Pharmaceutical What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. Effective risk management begins with a robust process to identify, quantify, and inventory risks, both familiar. An overall and continuing systematic process. What Is Risk Assessment In Pharma.
From www.slideserve.com
PPT Regulatory Compliance for Global Pharma Market PowerPoint What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk. What Is Risk Assessment In Pharma.
From www.getreskilled.com
Quality Risk Management in the Pharma View 8 QRM Tools What Is Risk Assessment In Pharma Develop a robust quantitative view of which risks matter most. An overall and continuing systematic process for the assessment, control, communication and. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards. What Is Risk Assessment In Pharma.
From www.americanpharmaceuticalreview.com
Environmental Monitoring Performance Qualification (EMPQ) Risk What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. 2.1 the holder of a manufacturing authorisation must manufacture medicinal products so as to ensure that they are fit for. An overall and continuing systematic process for the assessment, control, communication and. Risk assessment consists of the identification of hazards and the. What Is Risk Assessment In Pharma.
From www.slideteam.net
Mixing Process Risk Assessment Pharmaceutical Development New Medicine What Is Risk Assessment In Pharma This document provides principles and examples of tools for quality risk management that can be applied to different. Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. An overall and continuing systematic process for the assessment, control, communication and. Develop a robust quantitative view of which risks. What Is Risk Assessment In Pharma.
From www.slideteam.net
Initial Risk Assessment Of Manufacturing Process Steps Pharmaceutical What Is Risk Assessment In Pharma Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. Acquire sufficient product and process knowledge to assess risks associated with formulation development of the finished pharmaceutical. This document provides principles and examples of tools for quality risk management that. What Is Risk Assessment In Pharma.
From www.semanticscholar.org
Critical Risk Assessment and Management in Pharmaceutical Industry What Is Risk Assessment In Pharma Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as. Risk assessment, as it is described in ich q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process. 2.1 the holder of a manufacturing authorisation must. What Is Risk Assessment In Pharma.