What Is A Triple Bond Apex . Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. A triple bond is made of two pi bonds and one sigma bond. In a triple bond, two are pi bonds. In turn, electrons in a triple bond occupy more space. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. Count the number of regions of electron density (lone pairs and bonds) around the central atom. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. A single, double, or triple bond counts as one. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond;
from www.perplexity.ai
A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; Count the number of regions of electron density (lone pairs and bonds) around the central atom. A single, double, or triple bond counts as one. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A triple bond is made of two pi bonds and one sigma bond. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. In turn, electrons in a triple bond occupy more space. In a triple bond, two are pi bonds. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond.
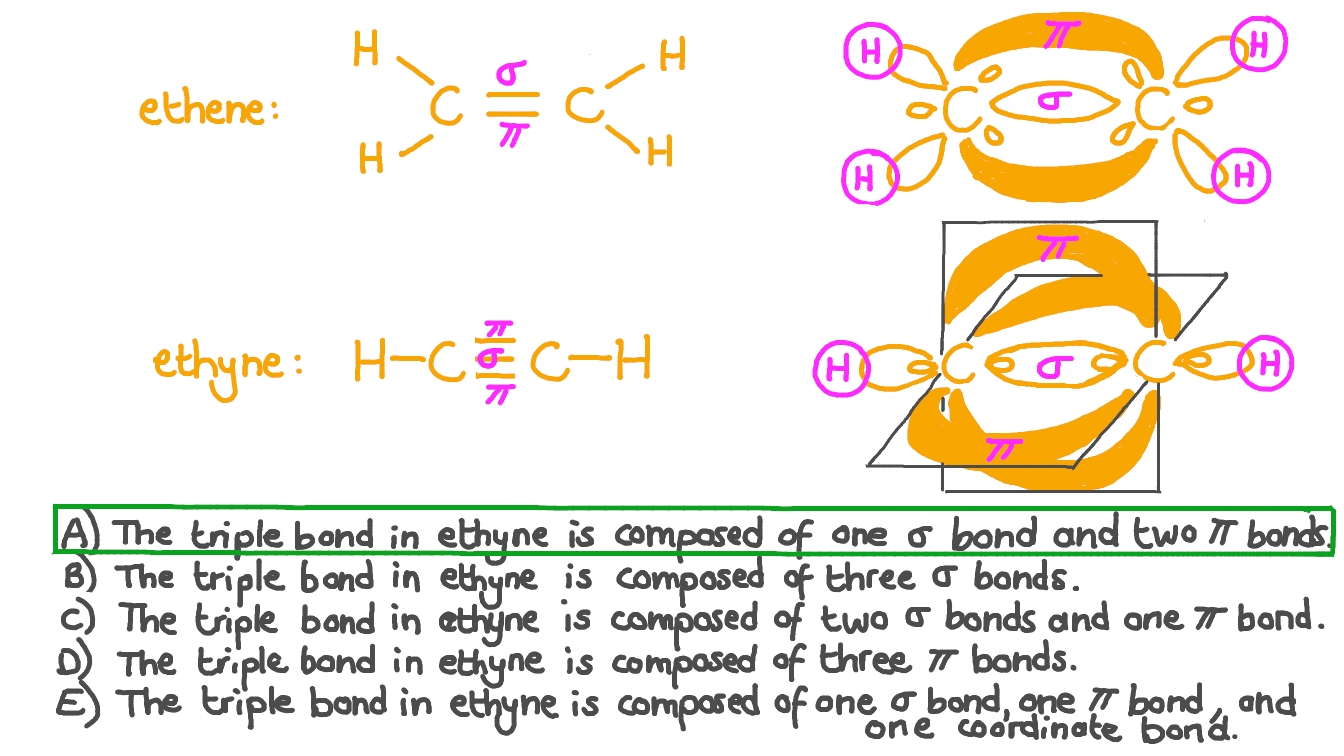
Help me with Hybridisation in a levels chemistry in context to 2 (a
What Is A Triple Bond Apex The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. Count the number of regions of electron density (lone pairs and bonds) around the central atom. In turn, electrons in a triple bond occupy more space. A single, double, or triple bond counts as one. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; In a triple bond, two are pi bonds. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. A triple bond is made of two pi bonds and one sigma bond. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms.
From www.slideserve.com
PPT Valence Bond Theory PowerPoint Presentation, free download ID What Is A Triple Bond Apex A single, double, or triple bond counts as one. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. Count the number of regions of electron density (lone. What Is A Triple Bond Apex.
From slideplayer.com
VSEPR and CARBON CHEMISTRY ppt download What Is A Triple Bond Apex Count the number of regions of electron density (lone pairs and bonds) around the central atom. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A triple bond is made of two pi bonds and one sigma bond. In turn, electrons in a. What Is A Triple Bond Apex.
From www.expii.com
Multiple Bonds — Double & Triple Bonds Expii What Is A Triple Bond Apex A triple bond is made of two pi bonds and one sigma bond. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; The covalent bond. What Is A Triple Bond Apex.
From www.slideserve.com
PPT Valence Bond Theory PowerPoint Presentation, free download ID What Is A Triple Bond Apex Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. A triple bond is made of two pi bonds and one sigma bond. In turn, electrons in a triple bond occupy. What Is A Triple Bond Apex.
From quizlet.com
Chapter 9 Triple bond Reagents I) Diagram Quizlet What Is A Triple Bond Apex A single, double, or triple bond counts as one. A triple bond is made of two pi bonds and one sigma bond. In a triple bond, two are pi bonds. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. Count the number of. What Is A Triple Bond Apex.
From stock.adobe.com
Covalent bonds [Single, double, triple] Stock Vector Adobe Stock What Is A Triple Bond Apex The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. In a triple bond, two are pi bonds. A lone pair of electrons occupies a larger region of space than. What Is A Triple Bond Apex.
From studiousguy.com
Triple Bond Examples StudiousGuy What Is A Triple Bond Apex Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. Count the number of regions of electron density (lone pairs and bonds) around the central atom. Octyne is. What Is A Triple Bond Apex.
From brainly.in
what is triple Bond or triple covalent bond explain the formation of What Is A Triple Bond Apex A single, double, or triple bond counts as one. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. Examples of. What Is A Triple Bond Apex.
From slideplayer.com
Chapter 6 Section ppt download What Is A Triple Bond Apex In a triple bond, two are pi bonds. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. In turn, electrons in a triple bond occupy more. What Is A Triple Bond Apex.
From www.sliderbase.com
Sigma and Pi Bonds What Is A Triple Bond Apex The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. Count the number of regions of electron density (lone pairs and bonds) around the central atom. Examples of compounds with. What Is A Triple Bond Apex.
From wisc.pb.unizin.org
7.6 Molecular Structure and Polarity Chemistry What Is A Triple Bond Apex In a triple bond, two are pi bonds. Count the number of regions of electron density (lone pairs and bonds) around the central atom. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. In turn, electrons in a triple bond occupy more space.. What Is A Triple Bond Apex.
From www.youtube.com
Formation of Triple Covalent Bonds YouTube What Is A Triple Bond Apex A single, double, or triple bond counts as one. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; Count the number of regions of electron. What Is A Triple Bond Apex.
From selfstudy365.com
[SOLVED] Which of the following compounds has a triple bond? Self What Is A Triple Bond Apex In a triple bond, two are pi bonds. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. In turn, electrons in a triple bond occupy more space. According to this. What Is A Triple Bond Apex.
From river-has-craig.blogspot.com
Which Best Describes the Bond Shown Apex RiverhasCraig What Is A Triple Bond Apex In a triple bond, two are pi bonds. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. In turn, electrons in a triple bond occupy more space. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; A triple bond is made of two pi. What Is A Triple Bond Apex.
From study.com
Triple Bond Overview, Definition & Examples Lesson What Is A Triple Bond Apex According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. Count the number of regions of electron density (lone pairs and bonds) around the central atom. In. What Is A Triple Bond Apex.
From byjus.com
How many sigma and Pi bonds are in HCONHCH3? What Is A Triple Bond Apex In a triple bond, two are pi bonds. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. In turn, electrons in a triple bond occupy more space. Octyne is a type of hydrocarbon with. What Is A Triple Bond Apex.
From www.chemistrylearner.com
Triple Covalent Bond Definition and Examples What Is A Triple Bond Apex The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. In turn, electrons in a triple bond occupy more space. Count the number of regions of electron density (lone pairs. What Is A Triple Bond Apex.
From www.vecteezy.com
Molecular model of Nitrogen N2 chemical molecule with one triple bond What Is A Triple Bond Apex A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; In turn, electrons in a triple bond occupy more space. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple. What Is A Triple Bond Apex.
From surfguppy.com
Carbon to Carbon Single, Double & Triple Bonds Surfguppy What Is A Triple Bond Apex Count the number of regions of electron density (lone pairs and bonds) around the central atom. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a.. What Is A Triple Bond Apex.
From sansona.github.io
Bond Attributes What Is A Triple Bond Apex Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. In a triple bond, two are pi bonds. In turn, electrons in a triple bond occupy more space. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a.. What Is A Triple Bond Apex.
From www.youtube.com
STRUCTURE OF TRIPLE BOND YouTube What Is A Triple Bond Apex In a triple bond, two are pi bonds. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; In turn, electrons in a triple bond occupy. What Is A Triple Bond Apex.
From www.perplexity.ai
Help me with Hybridisation in a levels chemistry in context to 2 (a What Is A Triple Bond Apex The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. In a triple bond, two are pi bonds. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. In turn, electrons in a triple bond occupy more. What Is A Triple Bond Apex.
From quizlet.com
Chapter 9 Other Triple bond Reagents I) Diagram Quizlet What Is A Triple Bond Apex A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. A triple bond is made of two pi bonds and one sigma bond. Octyne is a type of hydrocarbon with a chain of eight carbon atoms. What Is A Triple Bond Apex.
From pubs.rsc.org
Globally stabilized bent carboncarbon triple bond by hydrogenfree What Is A Triple Bond Apex Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. In turn, electrons in a triple bond occupy more space. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; A single, double, or triple bond counts as one. A triple bond is made of two. What Is A Triple Bond Apex.
From pediaa.com
Difference Between Single Double and Triple Bonds Definition What Is A Triple Bond Apex A single, double, or triple bond counts as one. Count the number of regions of electron density (lone pairs and bonds) around the central atom. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. According to this model, valence electrons in the lewis structure form groups, which may consist of a single. What Is A Triple Bond Apex.
From www.mdpi.com
Molecules Free FullText Dimer Rhenium Tetrafluoride with a Triple What Is A Triple Bond Apex Count the number of regions of electron density (lone pairs and bonds) around the central atom. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A. What Is A Triple Bond Apex.
From sciencenotes.org
Single, Double, and Triple Bonds What Is A Triple Bond Apex A triple bond is made of two pi bonds and one sigma bond. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; Octyne is a type of hydrocarbon with a chain of eight carbon. What Is A Triple Bond Apex.
From www.youtube.com
Organic compounds with both double and triple bonds YouTube What Is A Triple Bond Apex A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. Count the number of regions of electron density (lone pairs and bonds) around the central atom. In turn, electrons. What Is A Triple Bond Apex.
From mavink.com
Triple Bond Orbitals What Is A Triple Bond Apex In turn, electrons in a triple bond occupy more space. A single, double, or triple bond counts as one. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; In a triple bond, two are pi bonds. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon. What Is A Triple Bond Apex.
From valeriadesnhferguson.blogspot.com
Describes Double and Triple Bonds Using Valence Bond Theory What Is A Triple Bond Apex Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. Count the number of regions of electron density (lone pairs and bonds) around the central atom. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. A single, double, or triple. What Is A Triple Bond Apex.
From www.dreamstime.com
Three Types of Covalent Bonds Including Single, Double, and Triple What Is A Triple Bond Apex A triple bond is made of two pi bonds and one sigma bond. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. Examples of compounds with triple bonds include nitrogen gas, the cyanide ion, acetylene and carbon monoxide. Octyne is a type of hydrocarbon with a chain of eight carbon atoms and. What Is A Triple Bond Apex.
From www.chemistrylearner.com
Single Covalent Bond Definition and Examples What Is A Triple Bond Apex A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; In a triple bond, two are pi bonds. The covalent bond between 2 atoms where each atom contribute 3 electrons is called a triple bond. Count the number of regions of electron density (lone pairs and bonds) around the central atom. According. What Is A Triple Bond Apex.
From byjus.com
How many sigma and Pi bonds are in HCONHCH3? What Is A Triple Bond Apex Octyne is a type of hydrocarbon with a chain of eight carbon atoms and a triple bond between two of the carbon atoms. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. In a triple bond, two are pi bonds. The covalent bond. What Is A Triple Bond Apex.
From gamesmartz.com
Triple Bond Definition Easy to Understand What Is A Triple Bond Apex According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A lone pair of electrons occupies a larger region of space than the electrons in a triple bond; In a triple bond, two are pi bonds. Count the number of regions of electron density. What Is A Triple Bond Apex.
From studiousguy.com
Triple Bond Examples StudiousGuy What Is A Triple Bond Apex In a triple bond, two are pi bonds. A triple bond is made of two pi bonds and one sigma bond. According to this model, valence electrons in the lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a. A lone pair of electrons occupies a larger region of space than the. What Is A Triple Bond Apex.