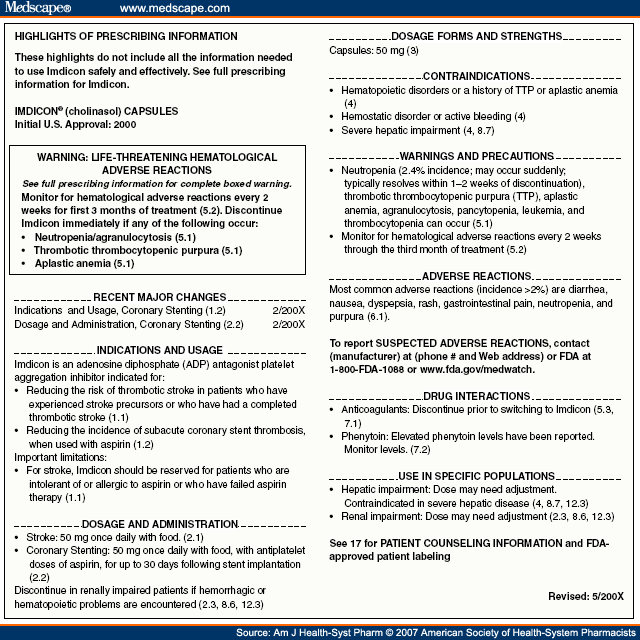
Fda Prescription Drug Labeling Requirements . “specific requirements on content and format of labeling for human prescription drugs; (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Addition of ‘geriatric use’ subsection in. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription.
from www.medscape.com
Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. “specific requirements on content and format of labeling for human prescription drugs; This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Addition of ‘geriatric use’ subsection in.
Introduction to the New Prescription Drug Labeling by the FDA
Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. (1) the labeling must contain a. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Addition of ‘geriatric use’ subsection in. “specific requirements on content and format of labeling for human prescription drugs; Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and.
From www.medscape.com
Introduction to the New Prescription Drug Labeling by the FDA Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. “specific requirements on content and format of labeling for human prescription drugs; Prescription drug labeling described in § 201.100 (d) must meet the. Fda Prescription Drug Labeling Requirements.
From www.dionlabel.com
Navigating FDA Labelling Requirements for CBD Products Fda Prescription Drug Labeling Requirements “specific requirements on content and format of labeling for human prescription drugs; On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Addition of ‘geriatric. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Addition of ‘geriatric use’ subsection in. “specific. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule. Fda Prescription Drug Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Prescription Drug Labeling Requirements “specific requirements on content and format of labeling for human prescription drugs; Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Addition of ‘geriatric use’ subsection in. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. “specific requirements on content and format of labeling for human prescription drugs; (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. On. Fda Prescription Drug Labeling Requirements.
From www.scribd.com
Labeling For Human Prescription Drug and Biological Products Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Addition of ‘geriatric use’ subsection in. “specific requirements on content and format of labeling for. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Addition of ‘geriatric use’ subsection in. (1) the labeling must contain a. This guidance is. Fda Prescription Drug Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Prescription Drug Labeling Requirements “specific requirements on content and format of labeling for human prescription drugs; Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content. Fda Prescription Drug Labeling Requirements.
From www.slideserve.com
PPT Overview of the New Content and Format Requirements for Fda Prescription Drug Labeling Requirements Addition of ‘geriatric use’ subsection in. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. “specific requirements on. Fda Prescription Drug Labeling Requirements.
From www.lifealert.org
OvertheCounter Medicine Label Fda Prescription Drug Labeling Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Addition of ‘geriatric use’ subsection in. Prescription drug labeling described in § 201.100 (d) must. Fda Prescription Drug Labeling Requirements.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Addition of. Fda Prescription Drug Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Prescription Drug Labeling Requirements (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist. Fda Prescription Drug Labeling Requirements.
From www.slideserve.com
PPT Labeling Prescription Drugs for Physicians and Consumers (FDA Fda Prescription Drug Labeling Requirements Addition of ‘geriatric use’ subsection in. (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Prescription drug labeling described in § 201.100 (d). Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. On january 24, 2006, the. Fda Prescription Drug Labeling Requirements.
From www.fda.gov
OTC Drug Facts Label FDA Fda Prescription Drug Labeling Requirements On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. “specific requirements on content and format of labeling for human prescription drugs; Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe. Fda Prescription Drug Labeling Requirements.
From blog.globalvision.co
Ensure Your Labels Meet all FDA Drug Labeling Requirements with Fda Prescription Drug Labeling Requirements “specific requirements on content and format of labeling for human prescription drugs; On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Addition of ‘geriatric use’ subsection in. (a) this part sets forth requirements for patient labeling for human. Fda Prescription Drug Labeling Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements Addition of ‘geriatric use’ subsection in. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health. Fda Prescription Drug Labeling Requirements.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Fda Prescription Drug Labeling Requirements (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. “specific requirements on content and format of labeling for human prescription drugs; (1) the labeling must contain a. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human. Fda Prescription Drug Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Prescription Drug Labeling Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. “specific requirements on content and format of labeling for human prescription drugs; Addition of ‘geriatric use’ subsection in. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a. This guidance is. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Addition of ‘geriatric use’ subsection in. On january 24, 2006, the food and drug. Fda Prescription Drug Labeling Requirements.
From www.slideserve.com
PPT Prescription Drug Labeling PowerPoint Presentation ID330259 Fda Prescription Drug Labeling Requirements Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and.. Fda Prescription Drug Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Prescription Drug Labeling Requirements “specific requirements on content and format of labeling for human prescription drugs; (1) the labeling must contain a. Addition of ‘geriatric use’ subsection in. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. (a) this. Fda Prescription Drug Labeling Requirements.
From www.fdalawblog.com
FDA Clarifies Labeling Expectations for Prescription Drug UseRelated Fda Prescription Drug Labeling Requirements This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Addition of ‘geriatric use’ subsection in. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. “specific. Fda Prescription Drug Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. “specific requirements on content and format of labeling for human prescription drugs; On. Fda Prescription Drug Labeling Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health. Fda Prescription Drug Labeling Requirements.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Fda Prescription Drug Labeling Requirements (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Addition of ‘geriatric use’ subsection in. “specific requirements on content and format of labeling for human prescription drugs; On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human. Fda Prescription Drug Labeling Requirements.
From www.bol.com
Content and Format of Labeling for Human Prescription Drug and Fda Prescription Drug Labeling Requirements Addition of ‘geriatric use’ subsection in. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: “specific requirements on content and format of labeling for human prescription drugs; Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. On january 24, 2006, the food and drug administration. Fda Prescription Drug Labeling Requirements.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Prescription Drug Labeling Requirements “specific requirements on content and format of labeling for human prescription drugs; Addition of ‘geriatric use’ subsection in. (1) the labeling must contain a. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. (a) this part sets forth. Fda Prescription Drug Labeling Requirements.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Prescription Drug Labeling Requirements Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription.. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements (1) the labeling must contain a. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. (a) this part sets forth requirements for patient labeling for human prescription drug products, including biological products, that the food and. Addition of ‘geriatric use’ subsection in. On january 24, 2006, the food and. Fda Prescription Drug Labeling Requirements.
From slidetodoc.com
An Introduction to the Improved FDA Prescription Drug Fda Prescription Drug Labeling Requirements Addition of ‘geriatric use’ subsection in. (1) the labeling must contain a. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. “specific requirements on content and format of labeling for human prescription drugs; Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription. Fda Prescription Drug Labeling Requirements.
From www.medscape.com
Introduction to the New Prescription Drug Labeling by the FDA Fda Prescription Drug Labeling Requirements This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. (1) the labeling must contain a. Human prescription drug. Fda Prescription Drug Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Prescription Drug Labeling Requirements On january 24, 2006, the food and drug administration (fda), an entity of the united states department of health and human services, published a final rule on the content and format. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Addition of ‘geriatric use’ subsection in. (1) the labeling must. Fda Prescription Drug Labeling Requirements.