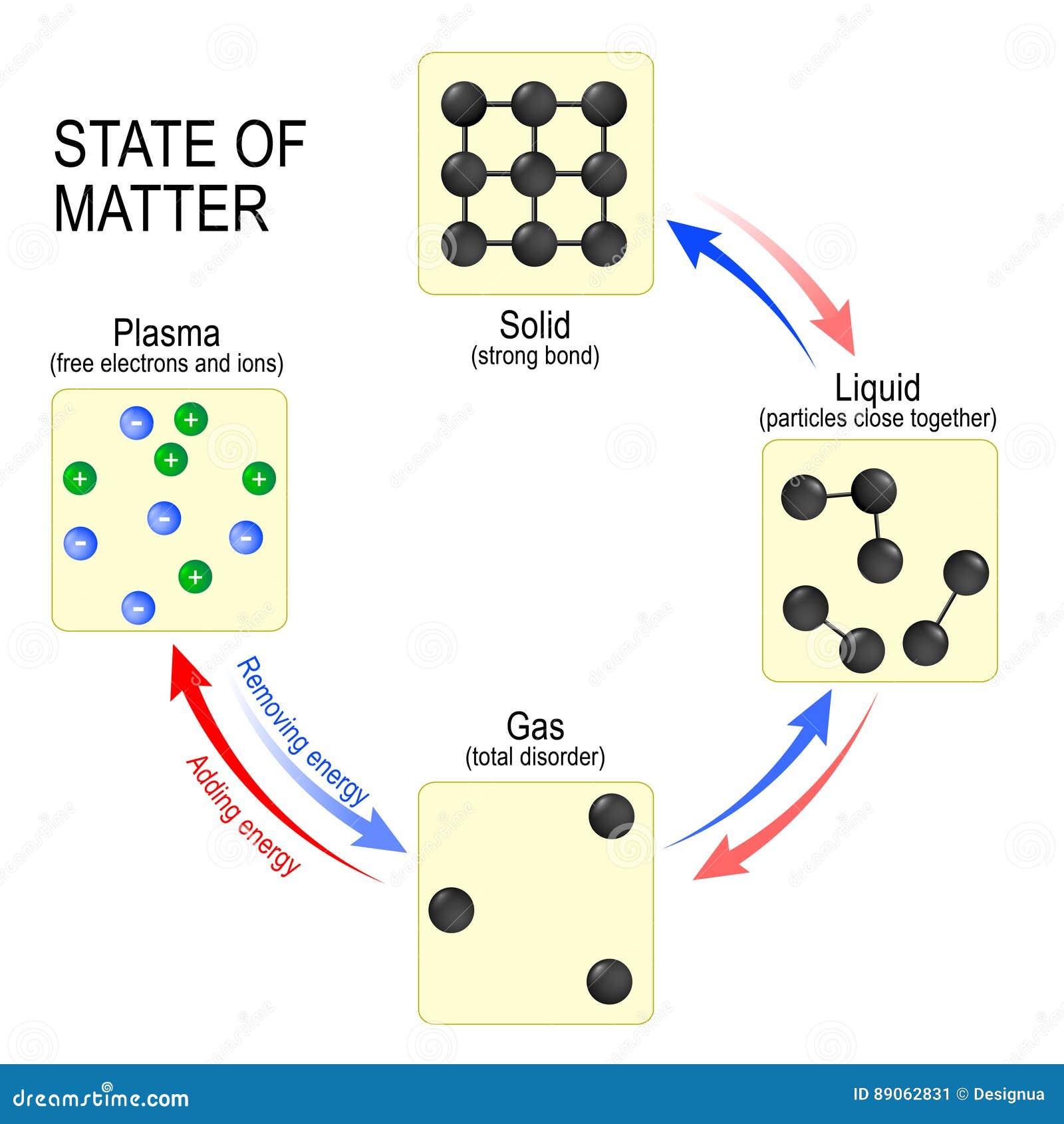
Solid To Gas To Liquid . Melting, freezing, evaporating and condensing. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). There are four main changes of state: The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids do not have to be. The substance changes from a gas to a liquid. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). Every element and substance can transition from one phase to another at a. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. When a liquid is cooled to even lower temperatures, it becomes a solid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state.
from www.dreamstime.com
The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The substance changes from a gas to a liquid. When a liquid is cooled to even lower temperatures, it becomes a solid. There are four main changes of state: Every element and substance can transition from one phase to another at a. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Melting, freezing, evaporating and condensing. Liquids do not have to be. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases.
States of Matter Solid, Liquid, Gas and Plasma. Stock Vector
Solid To Gas To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The substance changes from a gas to a liquid. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Every element and substance can transition from one phase to another at a. Liquids do not have to be. There are four main changes of state: When a liquid is cooled to even lower temperatures, it becomes a solid. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). Melting, freezing, evaporating and condensing.
From
Solid To Gas To Liquid The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. When a liquid is cooled to even lower temperatures, it becomes a solid. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Liquids do not have to be. The amount of energy required to. Solid To Gas To Liquid.
From
Solid To Gas To Liquid The substance changes from a gas to a liquid. Liquids do not have to be. There are four main changes of state: The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). Melting, freezing, evaporating and condensing. When a liquid is cooled to even lower temperatures, it becomes a solid.. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Liquids do not have to be. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). There are four main changes of state: Every element and substance can. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The substance changes from a gas to a liquid. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Every element and substance can transition from one phase to another at a. The substance changes from a gas to a liquid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to. Solid To Gas To Liquid.
From
Solid To Gas To Liquid There are four main changes of state: The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). Melting, freezing, evaporating and condensing. Every element and substance can transition from one phase to another. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Every element and substance can transition from one phase to another at a. There are four main changes of state: Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. When a liquid is cooled to even lower temperatures, it becomes a solid. The amount of energy required to sublime 1 mol. Solid To Gas To Liquid.
From
Solid To Gas To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Liquids do not have to be. The direct conversion of a solid to a gas, without an intervening. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Liquids do not have to be. Melting, freezing, evaporating and condensing. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. There are four main changes of state: The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). When a liquid is. Solid To Gas To Liquid.
From
Solid To Gas To Liquid There are four main changes of state: When a liquid is cooled to even lower temperatures, it becomes a solid. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and. Solid To Gas To Liquid.
From
Solid To Gas To Liquid When a liquid is cooled to even lower temperatures, it becomes a solid. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting, freezing, evaporating and condensing. The substance changes from a gas to a liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Liquids do not have to be. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The amount of energy required. Solid To Gas To Liquid.
From
Solid To Gas To Liquid The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. There are four main changes of state: The substance changes from a gas to a liquid. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. We take advantage of changes between the gas, liquid,. Solid To Gas To Liquid.
From
Solid To Gas To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). The substance changes from a gas to a liquid. The amount of energy required to sublime 1 mol. Solid To Gas To Liquid.
From stock.adobe.com
Vector diagram with changing states of matter, three states of matter Solid To Gas To Liquid There are four main changes of state: We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). The substance changes from a gas to a liquid. When a. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. When a liquid is cooled to even lower temperatures, it becomes a solid. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids do not have to be. Every element and substance can transition. Solid To Gas To Liquid.
From socratic.org
For the three states of matter (solid, liquid, and gas) there are six Solid To Gas To Liquid Liquids do not have to be. The substance changes from a gas to a liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. When a liquid is cooled to even lower temperatures, it becomes a solid. Heat, cool and compress atoms and molecules and watch as they change between solid,. Solid To Gas To Liquid.
From www.youtube.com
Heat and Phase Change Liquid to Gas to Liquid YouTube Solid To Gas To Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. There are four main changes of state: Every element and substance can transition from one phase to another at a. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Liquids do not have to. Solid To Gas To Liquid.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid To Gas To Liquid The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). When a liquid is cooled to even lower temperatures, it becomes a solid. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. We take advantage of changes between the gas, liquid,. Solid To Gas To Liquid.
From
Solid To Gas To Liquid When a liquid is cooled to even lower temperatures, it becomes a solid. Every element and substance can transition from one phase to another at a. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting, freezing, evaporating and condensing. The amount of energy required to sublime 1 mol of a pure. Solid To Gas To Liquid.
From
Solid To Gas To Liquid The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). The substance changes from a gas to a liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting, freezing, evaporating and condensing. Heat, cool and compress atoms and molecules and. Solid To Gas To Liquid.
From
Solid To Gas To Liquid There are four main changes of state: Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Every element and substance can transition from one phase to another at a. Liquids do not have to be. There are four main changes of state: The substance changes from a gas to a liquid. When a liquid is cooled to even. Solid To Gas To Liquid.
From
Solid To Gas To Liquid When a liquid is cooled to even lower temperatures, it becomes a solid. The substance changes from a gas to a liquid. Melting, freezing, evaporating and condensing. There are four main changes of state: The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. We take advantage of changes between the gas, liquid,. Solid To Gas To Liquid.
From
Solid To Gas To Liquid The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. There are four main changes of state: We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator. Solid To Gas To Liquid.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Solid To Gas To Liquid There are four main changes of state: When a liquid is cooled to even lower temperatures, it becomes a solid. Liquids do not have to be. The substance changes from a gas to a liquid. Melting, freezing, evaporating and condensing. Every element and substance can transition from one phase to another at a. We take advantage of changes between the. Solid To Gas To Liquid.
From
Solid To Gas To Liquid The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Melting, freezing, evaporating and condensing. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). Every element and substance can transition from one phase to another at a. The substance changes from a. Solid To Gas To Liquid.
From
Solid To Gas To Liquid There are four main changes of state: When a liquid is cooled to even lower temperatures, it becomes a solid. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Every element and substance can transition from one phase to another at a. We take advantage of changes between the gas, liquid, and. Solid To Gas To Liquid.
From
Solid To Gas To Liquid The substance changes from a gas to a liquid. Every element and substance can transition from one phase to another at a. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Melting, freezing, evaporating and condensing. The amount of energy required to sublime 1 mol of a pure solid is the. Solid To Gas To Liquid.
From
Solid To Gas To Liquid When a liquid is cooled to even lower temperatures, it becomes a solid. There are four main changes of state: The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting, freezing, evaporating and condensing.. Solid To Gas To Liquid.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Gas To Liquid Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The substance changes from a gas to a liquid. There are four main changes of state: Liquids do not have to be. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. Solid To Gas To Liquid.
From www.dreamstime.com
States of Matter . Solid , Liquid and Gas Vector Stock Vector Solid To Gas To Liquid There are four main changes of state: We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). The substance changes from a gas to a liquid. Every element. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Liquids do not have to be. When a liquid is cooled to even lower temperatures, it becomes a solid. Melting, freezing, evaporating and condensing. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). We take advantage of changes between the gas, liquid, and solid states to cool a drink. Solid To Gas To Liquid.
From
Solid To Gas To Liquid Melting, freezing, evaporating and condensing. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy of sublimation (δ hsub). The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. There are four main changes of state: The substance changes from a gas to a liquid. We take. Solid To Gas To Liquid.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Gas To Liquid Every element and substance can transition from one phase to another at a. Liquids do not have to be. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice. Solid To Gas To Liquid.