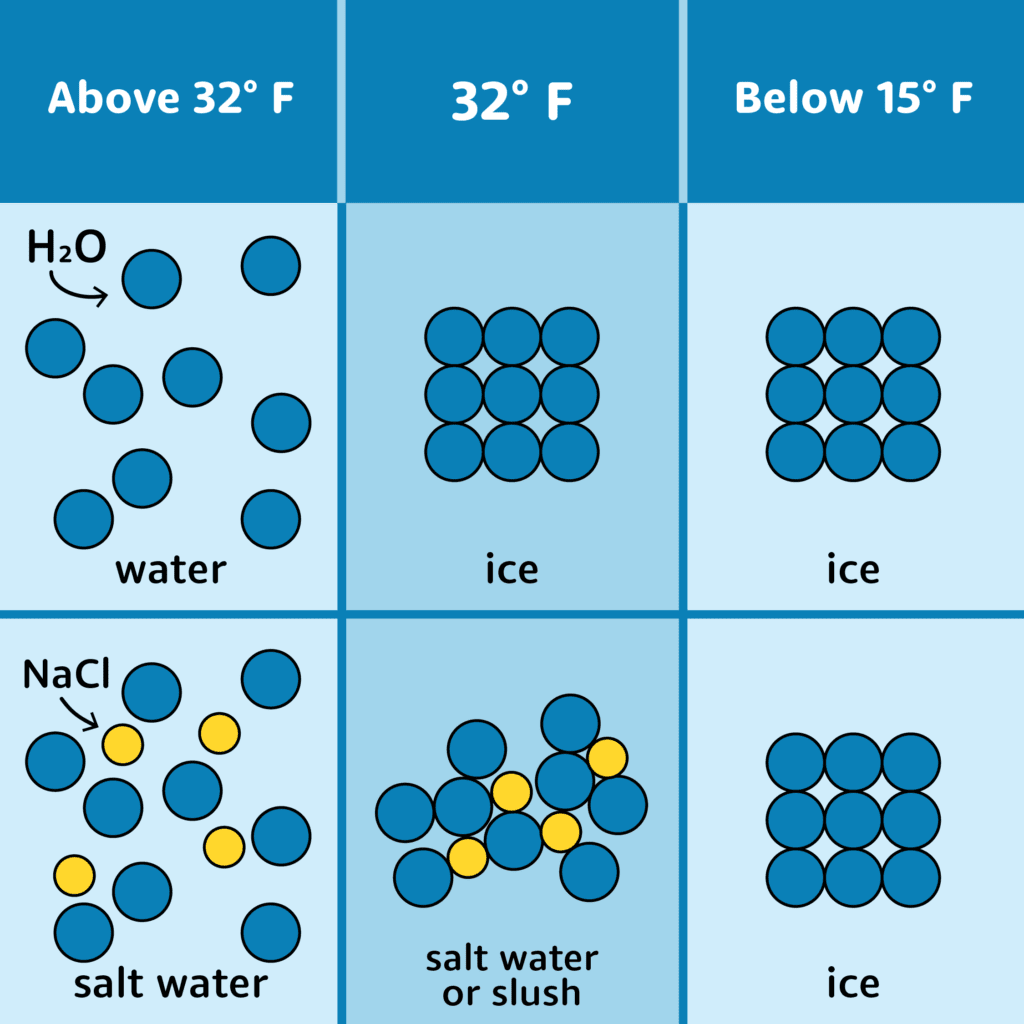
Can You Separate Salt From Water By Freezing Science Project . As you might already know, a salt water solution lowers the freezing point. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. In this experiment, students separate some of these salts from the mixture. The answer is simple — evaporation. Seawater is often called salt water but it contains various different salts. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. It's to get fresh water to drink. Yes, it is possible to separate salt from water, but only if you are careful. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. Among the available methods to.
from ldpwatersheds.org
Seawater is often called salt water but it contains various different salts. Yes, it is possible to separate salt from water, but only if you are careful. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. It's to get fresh water to drink. In this experiment, students separate some of these salts from the mixture. Among the available methods to. The answer is simple — evaporation. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. As you might already know, a salt water solution lowers the freezing point.
How Does Salt Melt Snow and Ice? LDP Watersheds
Can You Separate Salt From Water By Freezing Science Project You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. In this experiment, students separate some of these salts from the mixture. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. As you might already know, a salt water solution lowers the freezing point. Yes, it is possible to separate salt from water, but only if you are careful. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. Among the available methods to. Seawater is often called salt water but it contains various different salts. The answer is simple — evaporation. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. It's to get fresh water to drink.
From www.youtube.com
To Separate a Mixture of Sand and Water by Sedimentation and Can You Separate Salt From Water By Freezing Science Project In this experiment, students separate some of these salts from the mixture. The answer is simple — evaporation. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. When you cause saltwater to evaporate (either naturally or with artificial heat), only the. Can You Separate Salt From Water By Freezing Science Project.
From www.youtube.com
Science Experiments To separate common salt and sand from their Can You Separate Salt From Water By Freezing Science Project In this experiment, students separate some of these salts from the mixture. The answer is simple — evaporation. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. Seawater is often called salt water but it contains various different salts. As you might already know, a. Can You Separate Salt From Water By Freezing Science Project.
From www.pinterest.com
3 Ways to Separate Salt from Water Can You Separate Salt From Water By Freezing Science Project As you might already know, a salt water solution lowers the freezing point. Among the available methods to. Seawater is often called salt water but it contains various different salts. In this experiment, students separate some of these salts from the mixture. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there. Can You Separate Salt From Water By Freezing Science Project.
From melscience.com
“Salting out” experiment MEL Chemistry Can You Separate Salt From Water By Freezing Science Project Among the available methods to. As you might already know, a salt water solution lowers the freezing point. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. The answer is simple — evaporation. Seawater is often called salt water but. Can You Separate Salt From Water By Freezing Science Project.
From ldpwatersheds.org
How Does Salt Melt Snow and Ice? LDP Watersheds Can You Separate Salt From Water By Freezing Science Project You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. The answer is. Can You Separate Salt From Water By Freezing Science Project.
From www.alamy.com
Saline solution salt liquid water Stock Vector Images Alamy Can You Separate Salt From Water By Freezing Science Project As you might already know, a salt water solution lowers the freezing point. The answer is simple — evaporation. Among the available methods to. In this experiment, students separate some of these salts from the mixture. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either. Can You Separate Salt From Water By Freezing Science Project.
From www.thoughtco.com
How to Separate Salt and Sand — 3 Methods Can You Separate Salt From Water By Freezing Science Project Among the available methods to. Seawater is often called salt water but it contains various different salts. It's to get fresh water to drink. Yes, it is possible to separate salt from water, but only if you are careful. As you might already know, a salt water solution lowers the freezing point. The answer is simple — evaporation. You can. Can You Separate Salt From Water By Freezing Science Project.
From engl4kid.blogspot.com
English A2/B1 ноября 2020 Can You Separate Salt From Water By Freezing Science Project Among the available methods to. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. The answer is simple — evaporation. In this experiment, students separate some of these salts from the mixture. Seawater is often called salt water but it. Can You Separate Salt From Water By Freezing Science Project.
From byjus.com
How will you separate oil and water from their mixture? Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. When you cause. Can You Separate Salt From Water By Freezing Science Project.
From stanley-kbryant.blogspot.com
Why Does Salt Melt Ice Faster Than Sand Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. Among the available methods to. It's to get fresh water to drink. The answer is simple — evaporation. Yes, it is possible to separate salt from water, but only if you are. Can You Separate Salt From Water By Freezing Science Project.
From www.thoughtco.com
How to Separate Salt and Water Can You Separate Salt From Water By Freezing Science Project Yes, it is possible to separate salt from water, but only if you are careful. As you might already know, a salt water solution lowers the freezing point. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. In this experiment, students. Can You Separate Salt From Water By Freezing Science Project.
From www.nutriinspector.com
Can Salt Water Freeze Exploring the Salt's Frozen Depths Nutri Inspector Can You Separate Salt From Water By Freezing Science Project Yes, it is possible to separate salt from water, but only if you are careful. It's to get fresh water to drink. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. In simple terms, there isn't any space in the. Can You Separate Salt From Water By Freezing Science Project.
From recoveryranger.com
How Do You Separate Alcohol And Water? Recovery Ranger Can You Separate Salt From Water By Freezing Science Project You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. Among the available methods to. As you might already know, a salt water solution lowers the freezing point. In simple terms, there isn't any space in the ice crystal lattice for. Can You Separate Salt From Water By Freezing Science Project.
From picimgr.blogspot.com
Separation Of Sand Salt And Iron Filings Lab Answers small images Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. In this experiment, students separate some of these salts from the mixture. The answer is simple — evaporation. It's to get fresh water to drink. Seawater is often called salt water but. Can You Separate Salt From Water By Freezing Science Project.
From www.playosmo.com
How To Separate Salt And Water? DIY Science Project Ideas Can You Separate Salt From Water By Freezing Science Project You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. In this experiment, students separate some of these salts from the mixture. Yes, it is possible to separate salt from water, but only if you are careful. Among the available methods. Can You Separate Salt From Water By Freezing Science Project.
From mad-science.wonderhowto.com
Supercool Science Trick How to Turn Water into Ice on Command « Mad Can You Separate Salt From Water By Freezing Science Project As you might already know, a salt water solution lowers the freezing point. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor. Can You Separate Salt From Water By Freezing Science Project.
From www.preproom.org
Sand and Salt Separation Science Practical Expiriment used in School Can You Separate Salt From Water By Freezing Science Project The answer is simple — evaporation. Among the available methods to. In this experiment, students separate some of these salts from the mixture. It's to get fresh water to drink. Seawater is often called salt water but it contains various different salts. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue,. Can You Separate Salt From Water By Freezing Science Project.
From www.toppr.com
You are provided with a mixture of salt, sand, oil and water. Write the Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. Among the available methods to. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. You can also. Can You Separate Salt From Water By Freezing Science Project.
From www.flickr.com
Does Salt Water Freeze Pictures for Emily's Science Fair p… Flickr Can You Separate Salt From Water By Freezing Science Project Among the available methods to. Seawater is often called salt water but it contains various different salts. In this experiment, students separate some of these salts from the mixture. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. It's to get fresh water to drink.. Can You Separate Salt From Water By Freezing Science Project.
From www.flexiprep.com
NCERT Class VI Science Solutions Chapter 5 Separation of Substances Can You Separate Salt From Water By Freezing Science Project You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. The answer is simple — evaporation. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of. Can You Separate Salt From Water By Freezing Science Project.
From byjus.com
2. How to separate sugar and water solution? Can You Separate Salt From Water By Freezing Science Project The answer is simple — evaporation. Among the available methods to. It's to get fresh water to drink. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. Yes, it is possible to separate salt from water, but only if you. Can You Separate Salt From Water By Freezing Science Project.
From www.pinterest.com
13 Saltwater Experiment Easy science projects, Science experiments Can You Separate Salt From Water By Freezing Science Project Yes, it is possible to separate salt from water, but only if you are careful. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. As you might already know, a salt water solution lowers the freezing point. Seawater is often. Can You Separate Salt From Water By Freezing Science Project.
From www.tes.com
Separate Salt from Water STEM Activity STEM Experiment Teaching Can You Separate Salt From Water By Freezing Science Project The answer is simple — evaporation. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. In. Can You Separate Salt From Water By Freezing Science Project.
From www.sciencekiddo.com
Salt Water Experiment Ocean Science for Kids • The Science Kiddo Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. Seawater is often. Can You Separate Salt From Water By Freezing Science Project.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube Can You Separate Salt From Water By Freezing Science Project The answer is simple — evaporation. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. Seawater is often called salt water but it contains various different salts. Among the available methods to. It's to get fresh water to drink. In this experiment, students separate some. Can You Separate Salt From Water By Freezing Science Project.
From ar.inspiredpencil.com
Salt Dissolving In Water Can You Separate Salt From Water By Freezing Science Project As you might already know, a salt water solution lowers the freezing point. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. Yes, it is possible to separate salt from water, but only if you are careful. It's to get. Can You Separate Salt From Water By Freezing Science Project.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Can You Separate Salt From Water By Freezing Science Project Among the available methods to. The answer is simple — evaporation. Seawater is often called salt water but it contains various different salts. In this experiment, students separate some of these salts from the mixture. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of. Can You Separate Salt From Water By Freezing Science Project.
From www.freebsdarm.org
How Turn Water Into Ice She Males Free Videos Can You Separate Salt From Water By Freezing Science Project In this experiment, students separate some of these salts from the mixture. Yes, it is possible to separate salt from water, but only if you are careful. Seawater is often called salt water but it contains various different salts. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt. Can You Separate Salt From Water By Freezing Science Project.
From materialmcgheepearter.z21.web.core.windows.net
Separation Of Salt From Water Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. Yes, it is possible to separate salt from. Can You Separate Salt From Water By Freezing Science Project.
From sciencenotes.org
How to Separate Salt and Sugar Can You Separate Salt From Water By Freezing Science Project In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. Seawater is often called salt water but it contains various different salts. Yes, it is possible to separate salt from water, but only if you are careful. The answer is simple —. Can You Separate Salt From Water By Freezing Science Project.
From materialmcgheepearter.z21.web.core.windows.net
Separate Salt From Water Can You Separate Salt From Water By Freezing Science Project Seawater is often called salt water but it contains various different salts. In this experiment, students separate some of these salts from the mixture. In simple terms, there isn't any space in the ice crystal lattice for the extra atoms and there is no way to plug either of the ions (or. Among the available methods to. The answer is. Can You Separate Salt From Water By Freezing Science Project.
From www.youtube.com
Salt, Sugar or Hot Water Which melts ice faster? YouTube Can You Separate Salt From Water By Freezing Science Project It's to get fresh water to drink. Yes, it is possible to separate salt from water, but only if you are careful. As you might already know, a salt water solution lowers the freezing point. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. You. Can You Separate Salt From Water By Freezing Science Project.
From musikterik.blogspot.com
How Can You Separate Sand And Water musikterik Can You Separate Salt From Water By Freezing Science Project Among the available methods to. Seawater is often called salt water but it contains various different salts. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor — the salt is left behind. As you might already know, a salt water solution lowers the freezing point. In simple terms, there isn't any. Can You Separate Salt From Water By Freezing Science Project.
From littlebinsforlittlehands.com
Freezing Water Experiment Little Bins for Little Hands Can You Separate Salt From Water By Freezing Science Project It's to get fresh water to drink. Among the available methods to. You can also evaporate the water (boil it if you’re in a hurry) and weigh the salty residue, but you may need a very sensitive scale or balance for. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water vapor —. Can You Separate Salt From Water By Freezing Science Project.
From www.youtube.com
Freezing Point of Water Experiment Chemistry The Good and the Can You Separate Salt From Water By Freezing Science Project The answer is simple — evaporation. Seawater is often called salt water but it contains various different salts. It's to get fresh water to drink. As you might already know, a salt water solution lowers the freezing point. Among the available methods to. When you cause saltwater to evaporate (either naturally or with artificial heat), only the water forms water. Can You Separate Salt From Water By Freezing Science Project.