How To Calculate Concentration Of Hcl Titration . Ma is the molarity of the acid, while mb is the molarity of the base. At the start of the titration the solution is. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Va and vb are the volumes of the acid. Calculate the amount of sodium hydroxide in moles. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate the concentration of the hydrochloric acid solution. M\] we could do this in. To work out an unknown concentration of 0.15 ml hcl: If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Ma × va = mb × vb. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. The method involves reacting a.
from www.numerade.com
Ma is the molarity of the acid, while mb is the molarity of the base. Ma × va = mb × vb. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The method involves reacting a. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Calculate the amount of sodium hydroxide in moles. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. At the start of the titration the solution is. M\] we could do this in. Va and vb are the volumes of the acid.
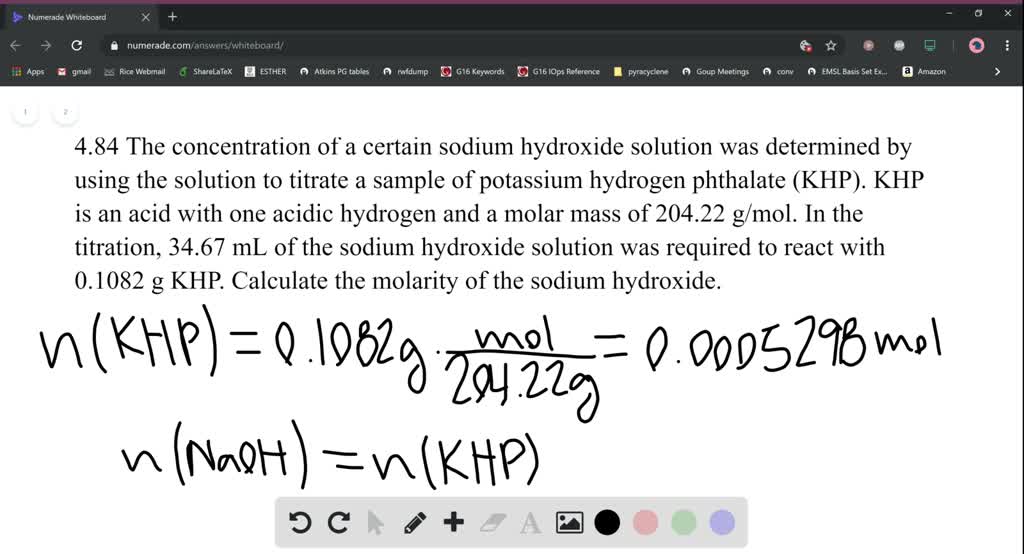
SOLVEDA solution of NaOH was standardized using solid KHP (fw = 204.32
How To Calculate Concentration Of Hcl Titration To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Ma is the molarity of the acid, while mb is the molarity of the base. Calculate the concentration of the hydrochloric acid solution. Calculate the amount of sodium hydroxide in moles. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). M\] we could do this in. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. The method involves reacting a. At the start of the titration the solution is. Ma × va = mb × vb. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Va and vb are the volumes of the acid. To work out an unknown concentration of 0.15 ml hcl:
From mungfali.com
Acid Base Titration Calculation How To Calculate Concentration Of Hcl Titration Ma is the molarity of the acid, while mb is the molarity of the base. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by. How To Calculate Concentration Of Hcl Titration.
From www.chegg.com
The data tables below are for a titration of an How To Calculate Concentration Of Hcl Titration To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. To work out an unknown concentration of 0.15 ml hcl: M\] we could. How To Calculate Concentration Of Hcl Titration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate Concentration Of Hcl Titration To work out an unknown concentration of 0.15 ml hcl: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate the concentration of the hydrochloric acid solution. M\] we could do this in. Calculate the amount of sodium hydroxide in moles. The method involves reacting a. If you know. How To Calculate Concentration Of Hcl Titration.
From www.nagwa.com
Question Video Calculating the Molar Concentration of Mg(OH)₂ Using How To Calculate Concentration Of Hcl Titration Va and vb are the volumes of the acid. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Calculate the amount of sodium hydroxide in moles. Calculate the concentration of the hydrochloric acid solution. The method involves reacting a. To calculate the. How To Calculate Concentration Of Hcl Titration.
From conorcierhan.blogspot.com
49+ how to calculate the hydroxide ion concentration ConorCierhan How To Calculate Concentration Of Hcl Titration Va and vb are the volumes of the acid. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Calculate the concentration of the hydrochloric acid solution. M\] we could do this in. At the start of the titration the solution is. Let's assume you are titrating a strong acid. How To Calculate Concentration Of Hcl Titration.
From ar.inspiredpencil.com
Titration Curve Acetic Acid How To Calculate Concentration Of Hcl Titration Ma is the molarity of the acid, while mb is the molarity of the base. Calculate the concentration of the hydrochloric acid solution. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Before the equivalence point, hcl is present in excess and. How To Calculate Concentration Of Hcl Titration.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate How To Calculate Concentration Of Hcl Titration At the start of the titration the solution is. To work out an unknown concentration of 0.15 ml hcl: Calculate the amount of sodium hydroxide in moles. Calculate the concentration of the hydrochloric acid solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Before the equivalence point, hcl. How To Calculate Concentration Of Hcl Titration.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Calculate Concentration Of Hcl Titration At the start of the titration the solution is. Calculate the concentration of the hydrochloric acid solution. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). To work out. How To Calculate Concentration Of Hcl Titration.
From mavink.com
Acid Base Titration Calculation How To Calculate Concentration Of Hcl Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Ma × va = mb × vb. At the start of the titration the solution is. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of. How To Calculate Concentration Of Hcl Titration.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via How To Calculate Concentration Of Hcl Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Ma is the molarity of the acid, while mb is the molarity of the base. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Calculate the concentration of the. How To Calculate Concentration Of Hcl Titration.
From www.youtube.com
Calculate Concentration (Example) YouTube How To Calculate Concentration Of Hcl Titration The method involves reacting a. To work out an unknown concentration of 0.15 ml hcl: If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Calculate the amount of sodium hydroxide in moles. At the start of the titration the solution is. Let's. How To Calculate Concentration Of Hcl Titration.
From solvedlib.com
Calculate the concentration of HCl acid if 50.00 mL o… SolvedLib How To Calculate Concentration Of Hcl Titration Va and vb are the volumes of the acid. To work out an unknown concentration of 0.15 ml hcl: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Ma is the molarity of the acid, while mb is the molarity of the base. The method involves reacting a. At. How To Calculate Concentration Of Hcl Titration.
From www.numerade.com
SOLVEDCalculate the concentration (in molarity) of an mathrm{NaOH How To Calculate Concentration Of Hcl Titration M\] we could do this in. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Calculate the concentration of the hydrochloric acid solution. Va and vb are the volumes of the acid. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m. How To Calculate Concentration Of Hcl Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Concentration Of Hcl Titration Calculate the concentration of the hydrochloric acid solution. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Ma is the molarity of the acid, while mb is the molarity of the base. To work out an unknown concentration of 0.15 ml hcl: Va and vb are the volumes of. How To Calculate Concentration Of Hcl Titration.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID How To Calculate Concentration Of Hcl Titration Ma × va = mb × vb. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). To work out an unknown concentration of 0.15 ml hcl: Calculate the amount. How To Calculate Concentration Of Hcl Titration.
From www.nagwa.com
Question Video Determining the Percentage of Base in a Mixture by How To Calculate Concentration Of Hcl Titration Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate the amount of sodium hydroxide in moles. At the start of the titration the solution is. Ma × va. How To Calculate Concentration Of Hcl Titration.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 How To Calculate Concentration Of Hcl Titration If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Ma is the molarity of the acid, while mb is the molarity of the base. The method involves reacting a. Ma × va = mb × vb. M\] we could do this in.. How To Calculate Concentration Of Hcl Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration Of Hcl Titration Calculate the concentration of the hydrochloric acid solution. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. M\] we could do this in. Calculate the amount of sodium hydroxide in moles. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of. How To Calculate Concentration Of Hcl Titration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution How To Calculate Concentration Of Hcl Titration To work out an unknown concentration of 0.15 ml hcl: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). At the start of the titration the solution is. M\] we could do this in. Calculate the concentration of the hydrochloric acid solution. Va and vb are the volumes of. How To Calculate Concentration Of Hcl Titration.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at How To Calculate Concentration Of Hcl Titration Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. Calculate the concentration of the hydrochloric acid solution. The method involves reacting a.. How To Calculate Concentration Of Hcl Titration.
From mungfali.com
Acid Base Titration Calculation How To Calculate Concentration Of Hcl Titration Ma × va = mb × vb. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). At the start of the titration the solution is. Ma is the molarity of the acid, while mb is the molarity of the base. Calculate the concentration of the hydrochloric acid solution. Va. How To Calculate Concentration Of Hcl Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How To Calculate Concentration Of Hcl Titration Ma is the molarity of the acid, while mb is the molarity of the base. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Calculate the amount of sodium hydroxide in moles. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m. How To Calculate Concentration Of Hcl Titration.
From morioh.com
Acid Base Titration Curves PH Calculations How To Calculate Concentration Of Hcl Titration Va and vb are the volumes of the acid. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Calculate the amount of sodium hydroxide in moles. Ma × va = mb × vb. To work out an unknown concentration of 0.15 ml hcl: At the start of the titration. How To Calculate Concentration Of Hcl Titration.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH How To Calculate Concentration Of Hcl Titration To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. At the start of the titration the solution is. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Ma is the molarity of the acid, while mb is the. How To Calculate Concentration Of Hcl Titration.
From www.chegg.com
Solved Sample Calculation A sample of 10.0 g of HCl solution How To Calculate Concentration Of Hcl Titration Ma is the molarity of the acid, while mb is the molarity of the base. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate the concentration of the hydrochloric acid solution. At the start of the titration the solution is. To calculate the concentration of the hcl solution,. How To Calculate Concentration Of Hcl Titration.
From www.numerade.com
SOLVEDA solution of NaOH was standardized using solid KHP (fw = 204.32 How To Calculate Concentration Of Hcl Titration At the start of the titration the solution is. M\] we could do this in. Va and vb are the volumes of the acid. Calculate the amount of sodium hydroxide in moles. The method involves reacting a. Ma × va = mb × vb. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00. How To Calculate Concentration Of Hcl Titration.
From boys.velvet.jp
Net Ionic Equation For Na2CO3 HCl Sodium Carbonate, 42 OFF How To Calculate Concentration Of Hcl Titration Calculate the concentration of the hydrochloric acid solution. At the start of the titration the solution is. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Calculate the amount of sodium hydroxide in moles. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. How To Calculate Concentration Of Hcl Titration.
From www.vrogue.co
Calculate The Molarity Of Naoh In The Solution Prepar vrogue.co How To Calculate Concentration Of Hcl Titration The method involves reacting a. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. M\] we could do this in. Ma is the molarity of the acid, while mb is the molarity of the base. To work out an unknown concentration of. How To Calculate Concentration Of Hcl Titration.
From www.numerade.com
SOLVED Write the laboratory report for determination of NaOH How To Calculate Concentration Of Hcl Titration If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. At the start of the titration the solution is. Ma × va = mb × vb. Calculate the concentration of the hydrochloric acid solution. Ma is the molarity of the acid, while mb. How To Calculate Concentration Of Hcl Titration.
From www.vrogue.co
Acid Base Titration Calculation Molar Concentration T vrogue.co How To Calculate Concentration Of Hcl Titration To work out an unknown concentration of 0.15 ml hcl: Va and vb are the volumes of the acid. Calculate the concentration of the hydrochloric acid solution. M\] we could do this in. The method involves reacting a. At the start of the titration the solution is. Ma is the molarity of the acid, while mb is the molarity of. How To Calculate Concentration Of Hcl Titration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Calculate Concentration Of Hcl Titration Calculate the concentration of the hydrochloric acid solution. To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. To work out an unknown concentration of 0.15 ml hcl: Va and vb are the volumes of the acid. Ma × va = mb × vb. If you know that titrating 50.00. How To Calculate Concentration Of Hcl Titration.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with How To Calculate Concentration Of Hcl Titration Ma is the molarity of the acid, while mb is the molarity of the base. To work out an unknown concentration of 0.15 ml hcl: The method involves reacting a. At the start of the titration the solution is. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate. How To Calculate Concentration Of Hcl Titration.
From www.youtube.com
The titration of a 10 00 mL sample of HCL solution of unknown How To Calculate Concentration Of Hcl Titration If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. At the start of the titration the solution is. To work out an unknown concentration of 0.15 ml hcl: Ma is the molarity of the acid, while mb is the molarity of the. How To Calculate Concentration Of Hcl Titration.
From mungfali.com
Acid Base Titration Formula How To Calculate Concentration Of Hcl Titration The method involves reacting a. If you know that titrating 50.00 ml of an hcl solution requires 25.00 ml of 1.00 m naoh, you can calculate the concentration of hydrochloric acid, hcl. At the start of the titration the solution is. Calculate the amount of sodium hydroxide in moles. To calculate the concentration of the hcl solution, we just divide. How To Calculate Concentration Of Hcl Titration.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration How To Calculate Concentration Of Hcl Titration To calculate the concentration of the hcl solution, we just divide the number of moles of hcl by the volume. Calculate the amount of sodium hydroxide in moles. Before the equivalence point, hcl is present in excess and the ph is determined by the concentration of unreacted hcl. Let's assume you are titrating a strong acid (10 ml unknown concentration. How To Calculate Concentration Of Hcl Titration.