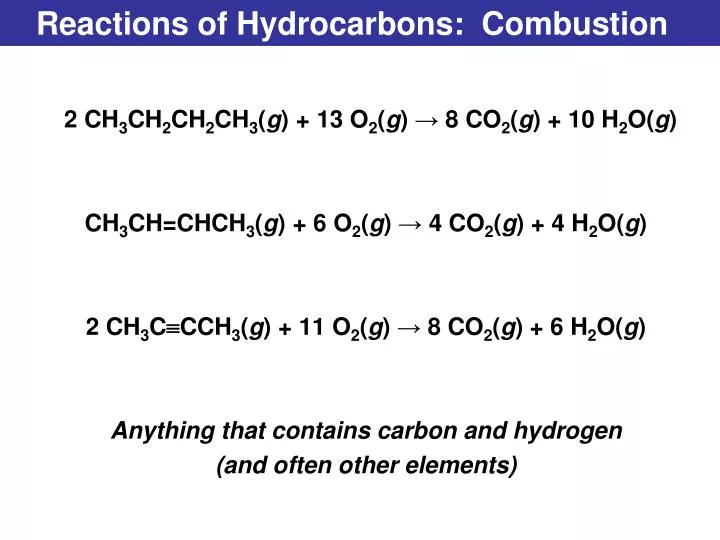
Burning Products Of Hydrocarbons . It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. Mineral matter turns into ash when burnt. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. fossil fuels are hydrocarbons comprised primarily of the following elements: many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). As all hydrocarbons only contain the elements carbon and. burning of hydrocarbons is an example of exothermic chemical reaction. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. Overall, the products of stoichiometric combustion of. combustion or burning is a high temperature exothermic reaction. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? hydrocarbon fuels burn when they react with oxygen in the air.
from www.slideserve.com
Overall, the products of stoichiometric combustion of. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? Mineral matter turns into ash when burnt. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). combustion or burning is a high temperature exothermic reaction. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. burning of hydrocarbons is an example of exothermic chemical reaction. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter.
PPT Reactions of Hydrocarbons Combustion PowerPoint Presentation
Burning Products Of Hydrocarbons Combustion is the chemical combination of a substance with oxygen, involving the production of heat. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. fossil fuels are hydrocarbons comprised primarily of the following elements: Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). As all hydrocarbons only contain the elements carbon and. hydrocarbon fuels burn when they react with oxygen in the air. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Mineral matter turns into ash when burnt. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. combustion or burning is a high temperature exothermic reaction. Overall, the products of stoichiometric combustion of. burning of hydrocarbons is an example of exothermic chemical reaction. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4?
From www.tes.com
Burning Hydrocarbon Fuels Teaching Resources Burning Products Of Hydrocarbons Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? combustion or burning is a high temperature exothermic reaction. Overall, the products of stoichiometric combustion of.. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT Reactions of Hydrocarbons Combustion PowerPoint Presentation Burning Products Of Hydrocarbons 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. burning of hydrocarbons is an example of exothermic chemical reaction. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. Water h 2 o (g) and carbon monoxide (co (g)) and/or. Burning Products Of Hydrocarbons.
From www.tes.com
Burning Hydrocarbon Fuels Teaching Resources Burning Products Of Hydrocarbons many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. hydrocarbon fuels burn when they react with oxygen in the air. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. As all hydrocarbons only contain the. Burning Products Of Hydrocarbons.
From www.bbc.co.uk
Combustion of hydrocarbon fuels The atmosphere OCR Gateway GCSE Burning Products Of Hydrocarbons hydrocarbon fuels burn when they react with oxygen in the air. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Overall,. Burning Products Of Hydrocarbons.
From www.youtube.com
Burning Hydrocarbons and the Keeling Curve YouTube Burning Products Of Hydrocarbons Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. hydrocarbon fuels burn when they react. Burning Products Of Hydrocarbons.
From www.dreamstime.com
Diagram of Hydrocarbon Combustion Stock Illustration Illustration of Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Mineral matter turns into ash when burnt. As all hydrocarbons only contain the elements carbon and. 9 ] ǃ b u001e qmj. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT The Combustion of Hydrocarbon Fuels PowerPoint Presentation, free Burning Products Of Hydrocarbons u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? Overall, the products of stoichiometric combustion of. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. hydrocarbon fuels burn when they react with oxygen in the air. fossil fuels are hydrocarbons comprised primarily of the following elements: burning. Burning Products Of Hydrocarbons.
From www.youtube.com
Burning Neste renewable hydrocarbons compared to fossil hydrocarbons Burning Products Of Hydrocarbons 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. combustion or burning is a high temperature exothermic reaction. burning of hydrocarbons is an example of exothermic chemical reaction. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). Overall, the products of stoichiometric combustion of. It happens between a fuel and. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT C1a 3 Crude oil PowerPoint Presentation, free download ID1711239 Burning Products Of Hydrocarbons As all hydrocarbons only contain the elements carbon and. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? Overall, the products of stoichiometric combustion of. fossil fuels are hydrocarbons comprised primarily of the following elements: 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. many combustion reactions occur with a hydrocarbon, a compound. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT Global Warming PowerPoint Presentation, free download ID6164833 Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. fossil fuels are hydrocarbons comprised primarily of the following elements: Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s),. Burning Products Of Hydrocarbons.
From www.teachit.co.uk
Burning hydrocarbons Flipped learning lesson KS4 chemistry Teachit Burning Products Of Hydrocarbons Mineral matter turns into ash when burnt. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). burning of hydrocarbons is an example of exothermic chemical reaction. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i.. Burning Products Of Hydrocarbons.
From www.youtube.com
Balancing Hydrocarbon Combustion Reactions YouTube Burning Products Of Hydrocarbons Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. fossil fuels are hydrocarbons comprised primarily of the following elements: Combustion is the chemical combination of a substance with oxygen, involving the production of heat. combustion or burning is a high temperature exothermic reaction. hydrocarbon fuels burn when they react with oxygen in the air. . Burning Products Of Hydrocarbons.
From slideplayer.com
Useful Products from Organic Sources ppt download Burning Products Of Hydrocarbons combustion or burning is a high temperature exothermic reaction. fossil fuels are hydrocarbons comprised primarily of the following elements: 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke.. Burning Products Of Hydrocarbons.
From slidetodoc.com
10 2 ALKANES Combustion of Alkanes The most Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). hydrocarbon fuels burn when they react with oxygen in the air. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. Mineral matter turns into ash when burnt. u0013 b hm u0001 bu001d> ~0u0007 u001du0012. Burning Products Of Hydrocarbons.
From byjus.com
Combustion Of Hydrocarbons Hydrocarbon Combustion Products Burning Products Of Hydrocarbons fossil fuels are hydrocarbons comprised primarily of the following elements: many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. combustion or burning is a high temperature exothermic reaction. burning of hydrocarbons is an example of exothermic chemical reaction. Mineral matter turns into ash when burnt. Carbon and hydrogen and some. Burning Products Of Hydrocarbons.
From www.alamy.com
Flame and smoke from the burning of hydrocarbons. Fossil fuels such as Burning Products Of Hydrocarbons Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. Overall, the products of stoichiometric combustion of. combustion or burning is a high temperature exothermic reaction. Mineral matter turns into ash when burnt. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r. Burning Products Of Hydrocarbons.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Burning Products Of Hydrocarbons It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). Mineral matter turns into ash when burnt. As all hydrocarbons only contain the elements carbon and. hydrocarbon fuels burn when they react with oxygen in the air. . Burning Products Of Hydrocarbons.
From mavink.com
Ejemplos De Combustion Burning Products Of Hydrocarbons As all hydrocarbons only contain the elements carbon and. hydrocarbon fuels burn when they react with oxygen in the air. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Overall, the products of stoichiometric combustion of. burning of hydrocarbons is an example of exothermic chemical reaction. combustion or burning is. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT The Atmosphere Chapter 17 PowerPoint Presentation, free download Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. fossil fuels are hydrocarbons comprised primarily of the following elements: As all hydrocarbons only contain the elements carbon and. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). Combustion is. Burning Products Of Hydrocarbons.
From www.tes.com
9.3 Burning Hydrocarbon fuels Teaching Resources Burning Products Of Hydrocarbons 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. burning of hydrocarbons is an example of exothermic chemical reaction. hydrocarbon fuels burn when they react with oxygen in the air. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon. Burning Products Of Hydrocarbons.
From www.learnaboutair.com
Combustion Learning about Air Quality Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). As all hydrocarbons only contain the elements carbon and. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed. Burning Products Of Hydrocarbons.
From www.youtube.com
of Hydrocarbons cyclohexane and cyclohexene. Alevel Burning Products Of Hydrocarbons Mineral matter turns into ash when burnt. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT L03. Burning hydrocarbons PowerPoint Presentation, free download Burning Products Of Hydrocarbons Mineral matter turns into ash when burnt. fossil fuels are hydrocarbons comprised primarily of the following elements: Overall, the products of stoichiometric combustion of. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? combustion or burning is a high temperature exothermic reaction. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. It happens. Burning Products Of Hydrocarbons.
From www.youtube.com
Demonstrating The Combustion Of Hydrocarbons YouTube Burning Products Of Hydrocarbons u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? As all hydrocarbons only contain the elements carbon and. hydrocarbon fuels burn when they react with oxygen in the air. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. It. Burning Products Of Hydrocarbons.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE Burning Products Of Hydrocarbons Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? hydrocarbon fuels burn when they react with oxygen in the air. Mineral matter turns into ash. Burning Products Of Hydrocarbons.
From www.tes.com
Burning Hydrocarbon Fuels Teaching Resources Burning Products Of Hydrocarbons burning of hydrocarbons is an example of exothermic chemical reaction. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. fossil fuels are hydrocarbons comprised primarily of the following elements: Mineral matter turns into ash when burnt.. Burning Products Of Hydrocarbons.
From slideplayer.com
Hydrocarbons. ppt download Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. Mineral matter turns into ash when burnt. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. combustion or burning is a high temperature exothermic reaction. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c. Burning Products Of Hydrocarbons.
From slideplayer.com
Y9 Chemistry unit lesson 9 ppt download Burning Products Of Hydrocarbons It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Overall, the products of stoichiometric combustion of. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i.. Burning Products Of Hydrocarbons.
From www.slideserve.com
PPT Hydrocarbons / Organic Chemistry PowerPoint Presentation, free Burning Products Of Hydrocarbons hydrocarbon fuels burn when they react with oxygen in the air. Overall, the products of stoichiometric combustion of. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. combustion or burning is a high temperature exothermic reaction. 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. burning of hydrocarbons. Burning Products Of Hydrocarbons.
From brainly.com
The diagram shows an experiment to investigate the products of burning Burning Products Of Hydrocarbons burning of hydrocarbons is an example of exothermic chemical reaction. fossil fuels are hydrocarbons comprised primarily of the following elements: u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i.. Burning Products Of Hydrocarbons.
From www.pinterest.com
ScienceSnaps 98 Burning of hydrocarbons. Photo credit and Location Burning Products Of Hydrocarbons Overall, the products of stoichiometric combustion of. Mineral matter turns into ash when burnt. u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? burning of hydrocarbons is an example of exothermic chemical reaction. Combustion is the chemical combination of a substance with oxygen, involving the production of heat. hydrocarbon fuels burn when they react with. Burning Products Of Hydrocarbons.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Burning Products Of Hydrocarbons Mineral matter turns into ash when burnt. fossil fuels are hydrocarbons comprised primarily of the following elements: 9 ] ǃ b u001e qmj ,ofu0002 6s2 r wu0011q`|mu001b i. It happens between a fuel and oxygen (oxidant), giving out gaseous products, also termed as smoke. Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. u0013 b hm. Burning Products Of Hydrocarbons.
From shop.sserltd.com
Atmosphere Effects of Burning Hydrocarbon Fuels SSER Ltd. Shop Burning Products Of Hydrocarbons u0013 b hm u0001 bu001d> ~0u0007 u001du0012 u001e t u00079 4? many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). Mineral matter turns into ash when burnt. As all hydrocarbons only contain the elements carbon and. It. Burning Products Of Hydrocarbons.
From www.youtube.com
Products of Hydrocarbon Combustion Experiment YouTube Burning Products Of Hydrocarbons many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. hydrocarbon fuels burn when they react with oxygen in the air. fossil fuels are hydrocarbons comprised primarily of the following elements: combustion or burning is a high temperature exothermic reaction. As all hydrocarbons only contain the elements carbon and. 9 ]. Burning Products Of Hydrocarbons.
From sterlingpixels.com
Combustion Reactions of Carbon Compounds Burning Products Of Hydrocarbons Water h 2 o (g) and carbon monoxide (co (g)) and/or carbon (c (s), soot). Carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. As all hydrocarbons only contain the elements carbon and. many combustion reactions occur with a hydrocarbon, a compound comprised solely of carbon and hydrogen. Combustion is the chemical combination of a substance with. Burning Products Of Hydrocarbons.