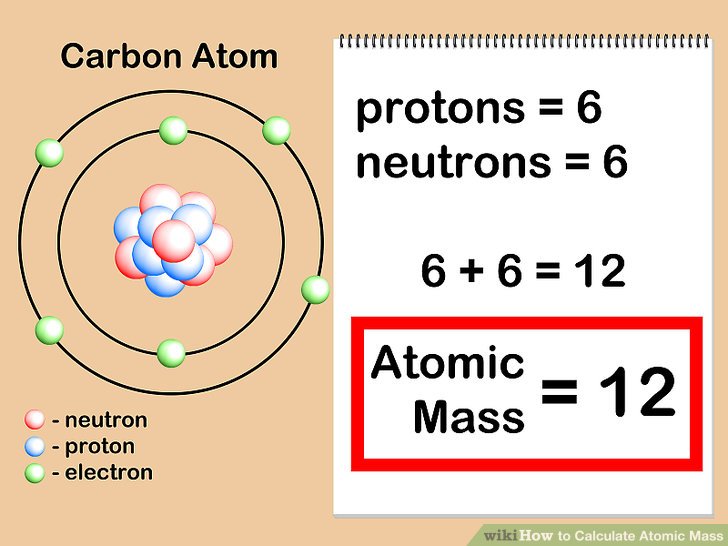
Atomic Number Average Mass . The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. So, how do we calculate the average. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The mass number of an atom is its total number. It has 11 positive charges and 11 negative charges. The relative atomic mass, ar, of an element is. Every sodium atom has 11 protons and 11 electrons.
from periodictable.me
So, how do we calculate the average. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. It has 11 positive charges and 11 negative charges. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The mass number of an atom is its total number. Every sodium atom has 11 protons and 11 electrons. The relative atomic mass, ar, of an element is.
Periodic Table Element With Atomic Mass And Atomic Number
Atomic Number Average Mass Every sodium atom has 11 protons and 11 electrons. The relative atomic mass, ar, of an element is. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. So, how do we calculate the average. Every sodium atom has 11 protons and 11 electrons. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. It has 11 positive charges and 11 negative charges. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The mass number of an atom is its total number. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Atomic Number Average Mass The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. It has 11 positive charges and 11 negative charges. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. The relative atomic mass,. Atomic Number Average Mass.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Atomic Number Average Mass Every sodium atom has 11 protons and 11 electrons. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. So, how do we calculate the average. The mass of an atom is a weighted average that is largely determined by the number of its protons. Atomic Number Average Mass.
From www.geeksforgeeks.org
Differences Between Atomic Mass and Atomic Number Atomic Number Average Mass The relative atomic mass, ar, of an element is. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. So,. Atomic Number Average Mass.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Atomic Number Average Mass The relative atomic mass, ar, of an element is. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. It has 11 positive charges and 11 negative charges. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass. Atomic Number Average Mass.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow Atomic Number Average Mass It has 11 positive charges and 11 negative charges. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The. Atomic Number Average Mass.
From www.slideserve.com
PPT Atomic Mass PowerPoint Presentation, free download ID3983503 Atomic Number Average Mass The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. The relative atomic mass, ar, of an element is. Every sodium atom has 11 protons and 11 electrons. So, how do we calculate the average. The mass number of an atom is its. Atomic Number Average Mass.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Atomic Number Average Mass The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. It has 11 positive charges and 11 negative charges. Every sodium atom has 11 protons and 11 electrons. The average atomic mass is the average mass of all the isotopes that compose that element, weighted. Atomic Number Average Mass.
From www.youtube.com
How To Calculate The Average Atomic Mass YouTube Atomic Number Average Mass The relative atomic mass, ar, of an element is. It has 11 positive charges and 11 negative charges. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. Every sodium atom has 11 protons and 11 electrons. The atomic number (represented by the letter z) of an element is the. Atomic Number Average Mass.
From www.youtube.com
Calculating average atomic mass of elements Tutorial YouTube Atomic Number Average Mass The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The mass number of an atom is its total number. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The mass of an atom. Atomic Number Average Mass.
From www.slideserve.com
PPT Calculating Average Atomic Mass PowerPoint Presentation, free Atomic Number Average Mass The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The mass number of an atom is its total number. Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges. The average atomic mass calculator determines the. Atomic Number Average Mass.
From pediaa.com
Difference Between Mass Number and Atomic Mass Atomic Number Average Mass It has 11 positive charges and 11 negative charges. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. Every. Atomic Number Average Mass.
From www.slideserve.com
PPT Chapter 7 Chemical Formula Relationships PowerPoint Presentation Atomic Number Average Mass The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. It has 11 positive charges and 11 negative charges. The. Atomic Number Average Mass.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Atomic Number Average Mass The relative atomic mass, ar, of an element is. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons. Atomic Number Average Mass.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Atomic Number Average Mass Every sodium atom has 11 protons and 11 electrons. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The relative atomic mass, ar, of an element is. The mass of an atom is a weighted average that is largely determined by the number of. Atomic Number Average Mass.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Atomic Number Average Mass The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. It has 11 positive charges and 11 negative charges. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The. Atomic Number Average Mass.
From hunterturninaing.blogspot.com
how to find the average atomic mass Hunter Turninaing Atomic Number Average Mass The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges. So, how do we calculate the average. The atomic number (represented by the letter z). Atomic Number Average Mass.
From www.youtube.com
Atomic Number & Atomic Mass (Mass Number) amu, Moles, Grams, Molar Atomic Number Average Mass The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The relative atomic mass, ar, of an element is. The. Atomic Number Average Mass.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Atomic Number Average Mass The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. The average atomic mass is the average mass. Atomic Number Average Mass.
From www.youtube.com
Atomic Mass vs Atomic Number YouTube Atomic Number Average Mass The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. It has 11 positive charges and 11 negative charges. The mass number of an atom is its total number. The atomic number (represented by the letter z) of an element is the number of protons. Atomic Number Average Mass.
From sciencenotes.org
Periodic Table with Atomic Mass Atomic Number Average Mass The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. The average atomic mass is the average mass of all the isotopes that. Atomic Number Average Mass.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Atomic Number Average Mass The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. Every sodium atom has 11 protons and 11 electrons. So, how do we calculate the average. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on. Atomic Number Average Mass.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples Atomic Number Average Mass It has 11 positive charges and 11 negative charges. So, how do we calculate the average. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The relative atomic mass, ar, of an element is. The atomic number (represented by the letter z) of an. Atomic Number Average Mass.
From howtofindbasic.blogspot.com
How To Find The Average Atomic Mass Of An Isotope Find Howtos Atomic Number Average Mass Every sodium atom has 11 protons and 11 electrons. The relative atomic mass, ar, of an element is. The mass number of an atom is its total number. It has 11 positive charges and 11 negative charges. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the. Atomic Number Average Mass.
From sciencenotes.org
Periodic Table with Atomic Mass Atomic Number Average Mass The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. It has 11 positive charges and 11 negative charges. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons. Atomic Number Average Mass.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID Atomic Number Average Mass Every sodium atom has 11 protons and 11 electrons. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The mass number of an atom. Atomic Number Average Mass.
From nursehub.com
Atomic Number and Atomic Mass NurseHub Atomic Number Average Mass The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The mass number of an atom is its total number. The relative atomic mass, ar, of an element is. So, how do we calculate the average. The mass of an atom is a weighted average that is largely determined by. Atomic Number Average Mass.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Atomic Number Average Mass The relative atomic mass, ar, of an element is. The mass number of an atom is its total number. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and. The average atomic mass calculator determines the average atomic mass of elements based on. Atomic Number Average Mass.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Atomic Number Average Mass So, how do we calculate the average. The relative atomic mass, ar, of an element is. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their. Atomic Number Average Mass.
From pubchemblog.ncbi.nlm.nih.gov
Atomic mass changes in PubChem PubChem Blog Atomic Number Average Mass The relative atomic mass, ar, of an element is. The mass number of an atom is its total number. Every sodium atom has 11 protons and 11 electrons. The average atomic mass calculator determines the average atomic mass of elements based on the isotopic mass and their natural. The mass of an atom is a weighted average that is largely. Atomic Number Average Mass.
From www.printablee.com
Periodic Table With Mass And Atomic Number 10 Free PDF Printables Atomic Number Average Mass It has 11 positive charges and 11 negative charges. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based on the natural abundance of each isotope. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. So,. Atomic Number Average Mass.
From creditroden.weebly.com
creditroden Blog Atomic Number Average Mass So, how do we calculate the average. The mass number of an atom is its total number. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The average atomic mass is the average mass of all the isotopes that compose that element, weighted based. Atomic Number Average Mass.
From www.studocu.com
Atomic Mass and Atomic Number Worksheet Key Studocu Atomic Number Average Mass The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. It has 11 positive charges and 11 negative charges. Every. Atomic Number Average Mass.
From www.sliderbase.com
Isotopes Atomic Number Average Mass Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges. The mass number of an atom is its total number. The relative atomic mass, ar, of an element is. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of. Atomic Number Average Mass.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Atomic Number Average Mass The mass number of an atom is its total number. Every sodium atom has 11 protons and 11 electrons. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of. The average atomic mass is the average mass of all the isotopes that compose that element,. Atomic Number Average Mass.
From www.thoughtco.com
Difference Between Atomic Mass and Mass Number Atomic Number Average Mass The mass number of an atom is its total number. The atomic number (represented by the letter z) of an element is the number of protons in the nucleus of each atom of that element.an. It has 11 positive charges and 11 negative charges. Every sodium atom has 11 protons and 11 electrons. So, how do we calculate the average.. Atomic Number Average Mass.