Copper(Ii) Sulfate Soluble Or Insoluble . Copper (ii) sulfate can be prepared in the. Or determine the solubility using the solubility rules. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Another good way is to reduce it with iron or zinc to. All sulfates are soluble except strontium sulfate and barium sulfate. Titration must be used if the reactants are soluble. The compound melts at 110°c (230°f) with decomposition. It is highly soluble in water. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Enter a chemical formula of a substance to find its solubility using our solubility calculator.
from www.dexform.com
Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Another good way is to reduce it with iron or zinc to. Soluble salts can be made by reacting acids with soluble or insoluble reactants. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Or determine the solubility using the solubility rules. Copper (ii) sulfate can be prepared in the. Titration must be used if the reactants are soluble. All sulfates are soluble except strontium sulfate and barium sulfate. Enter a chemical formula of a substance to find its solubility using our solubility calculator. It is highly soluble in water.
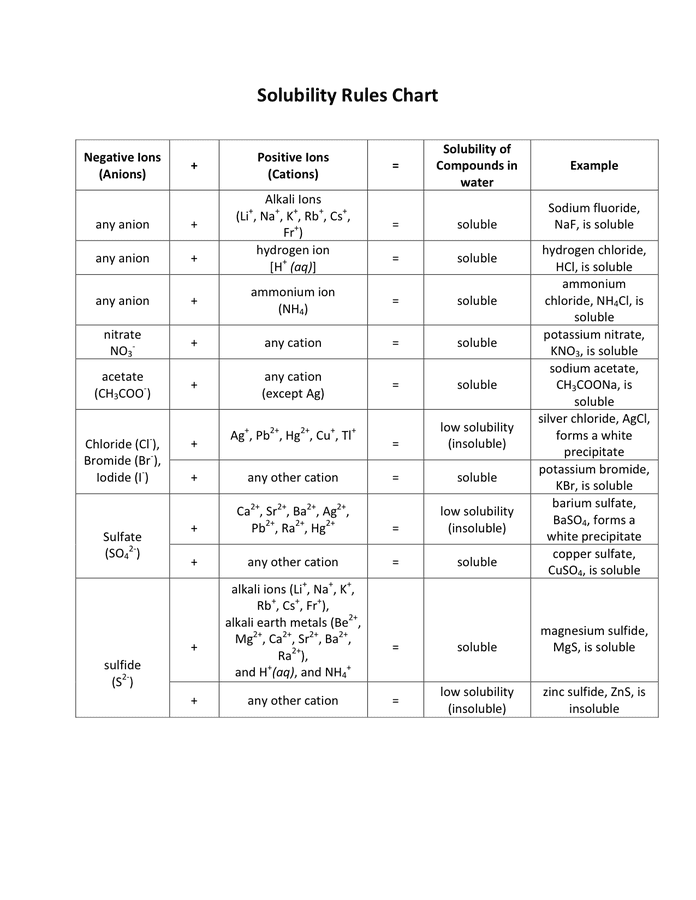
Solubility Rules Chart in Word and Pdf formats
Copper(Ii) Sulfate Soluble Or Insoluble Another good way is to reduce it with iron or zinc to. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. All sulfates are soluble except strontium sulfate and barium sulfate. Or determine the solubility using the solubility rules. The compound melts at 110°c (230°f) with decomposition. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Another good way is to reduce it with iron or zinc to. It is highly soluble in water. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Copper (ii) sulfate can be prepared in the.
From dxowpviqu.blob.core.windows.net
Tin(Iv) Sulfate Solubility at Celia Wilson blog Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Another good way is to reduce it with iron or zinc to. It is highly soluble in water. The compound melts at 110°c (230°f) with decomposition. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Enter a chemical formula of a substance to find its solubility. Copper(Ii) Sulfate Soluble Or Insoluble.
From mungfali.com
Solubility Rules Flowchart Chart Chemistry Copper(Ii) Sulfate Soluble Or Insoluble It is highly soluble in water. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Copper (ii) sulfate can be prepared in the. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. All sulfates are soluble except strontium sulfate and barium sulfate. The compound melts at 110°c (230°f) with decomposition. Copper sulfate can be disposed. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.pinterest.com.mx
Solubility Rules Chart for Chemistry Classroom Teaching chemistry Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. The compound melts at 110°c (230°f) with decomposition. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. All sulfates are. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.biomart.cn
Solubility Factors When Choosing a Solvent 企业动态 丁香通 Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Enter a chemical formula of a substance to find its solubility using our solubility calculator. The compound melts at 110°c (230°f) with decomposition. Or determine the. Copper(Ii) Sulfate Soluble Or Insoluble.
From surfguppy.com
Solubility Surfguppy Chemistry made easy for visual learners Copper(Ii) Sulfate Soluble Or Insoluble Copper (ii) sulfate can be prepared in the. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Titration must be used if the reactants are soluble. Another good way is to reduce it with iron or zinc to. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. The compound melts at 110°c (230°f). Copper(Ii) Sulfate Soluble Or Insoluble.
From www.sciencephoto.com
Copper (II) sulphate forms Stock Image C004/7693 Science Photo Copper(Ii) Sulfate Soluble Or Insoluble Another good way is to reduce it with iron or zinc to. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Or determine the solubility using the solubility rules. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble,. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.researchgate.net
Solubility diagram for different Cu(I) and Cu(II) solids likely in new Copper(Ii) Sulfate Soluble Or Insoluble Another good way is to reduce it with iron or zinc to. Soluble salts can be made by reacting acids with soluble or insoluble reactants. It is highly soluble in water. Enter a chemical formula of a substance to find its solubility using our solubility calculator. All sulfates are soluble except strontium sulfate and barium sulfate. Copper (ii) sulfate can. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.youtube.com
How to separate sand and copper(II) sulfate salt from its mixture Copper(Ii) Sulfate Soluble Or Insoluble The compound melts at 110°c (230°f) with decomposition. Enter a chemical formula of a substance to find its solubility using our solubility calculator. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Another good way is to reduce it with iron or zinc to. All sulfates are soluble except strontium sulfate and barium sulfate. Copper sulfate can be disposed. Copper(Ii) Sulfate Soluble Or Insoluble.
From niamhhenderson.z13.web.core.windows.net
Periodic Table With Solubility Chart Copper(Ii) Sulfate Soluble Or Insoluble Enter a chemical formula of a substance to find its solubility using our solubility calculator. Titration must be used if the reactants are soluble. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Copper (ii) sulfate can be prepared in the. The compound melts at. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.dexform.com
Solubility Rules Chart in Word and Pdf formats Copper(Ii) Sulfate Soluble Or Insoluble Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Enter a chemical formula of a substance to find its solubility using our solubility calculator. The compound melts at 110°c (230°f) with decomposition. Copper (ii) sulfate can be. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.wikidoc.org
Copper(II) sulfate wikidoc Copper(Ii) Sulfate Soluble Or Insoluble Copper (ii) sulfate can be prepared in the. It is highly soluble in water. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Enter a chemical. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.amazon.com
Copper (II) Sulfate Solution, 1M, 500mL The Curated Chemical Copper(Ii) Sulfate Soluble Or Insoluble The compound melts at 110°c (230°f) with decomposition. Titration must be used if the reactants are soluble. It is highly soluble in water. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. All sulfates are. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.tes.com
Making a Soluble Salt from an Insoluble Base (Copper Sulfate Core Copper(Ii) Sulfate Soluble Or Insoluble Another good way is to reduce it with iron or zinc to. Or determine the solubility using the solubility rules. Copper (ii) sulfate can be prepared in the. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a. Copper(Ii) Sulfate Soluble Or Insoluble.
From chemicaldb.netlify.app
Copper ii sulfate solubility Copper(Ii) Sulfate Soluble Or Insoluble Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing. Copper(Ii) Sulfate Soluble Or Insoluble.
From chemicaldb.netlify.app
Copper ii sulfate solubility Copper(Ii) Sulfate Soluble Or Insoluble Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Titration must be used if the reactants are soluble. It is highly soluble in water. All sulfates are soluble except strontium sulfate and barium sulfate. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Copper (ii) sulfate. Copper(Ii) Sulfate Soluble Or Insoluble.
From mungfali.com
Solubility Chemistry Diagram Copper(Ii) Sulfate Soluble Or Insoluble Copper (ii) sulfate can be prepared in the. It is highly soluble in water. Soluble salts can be made by reacting acids with soluble or insoluble reactants. All sulfates are soluble except strontium sulfate and barium sulfate. Another good way is to reduce it with iron or zinc to. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. The. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.sciencephoto.com
Hydrated copper (II) sulphate Stock Image C009/9431 Science Photo Copper(Ii) Sulfate Soluble Or Insoluble Copper (ii) sulfate can be prepared in the. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. It is highly soluble in water. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Titration must be used if the reactants are soluble. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1]. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.anglesandacid.com
Precipitation Reactions Copper(Ii) Sulfate Soluble Or Insoluble Copper (ii) sulfate can be prepared in the. Another good way is to reduce it with iron or zinc to. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Or determine the solubility using the solubility rules. Enter a chemical formula of a substance to find its solubility using our. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.pathwaystochemistry.com
Precipitation Reactions Pathways to Chemistry Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Another good way is to reduce it with iron or zinc to. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Or determine the solubility using the solubility rules. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.sciencephoto.com
Copper (II) Sulphate Stock Image C036/3376 Science Photo Library Copper(Ii) Sulfate Soluble Or Insoluble It is highly soluble in water. The compound melts at 110°c (230°f) with decomposition. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. All sulfates are soluble except strontium sulfate and barium sulfate. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Or determine the solubility using the solubility. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.sciencephoto.com
Copper sulphate solution Stock Image C008/7782 Science Photo Library Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Copper (ii) sulfate can be prepared in the. Titration must be used if the reactants are soluble. The compound melts at 110°c (230°f) with decomposition. Another good way is to reduce it with iron or zinc to. Or determine the solubility using the solubility rules. Enter a chemical formula of. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.numerade.com
SOLVED Soluble Insoluble magnesium sulfate sodium sulfate copper(II Copper(Ii) Sulfate Soluble Or Insoluble Soluble salts can be made by reacting acids with soluble or insoluble reactants. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. 133 rows all carbonates,. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.expii.com
Soluble vs. Insoluble — Comparison & Examples Expii Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. It is highly soluble in water. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Titration must be used if. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.rcilabscan.com
Copper (II) Sulfate Pentahydrate, AR RCI LABSCAN LIMITED (EN) Copper(Ii) Sulfate Soluble Or Insoluble Titration must be used if the reactants are soluble. Copper (ii) sulfate can be prepared in the. All sulfates are soluble except strontium sulfate and barium sulfate. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Another good way is to reduce it with iron or zinc to. Copper sulfate can be disposed of by either. Copper(Ii) Sulfate Soluble Or Insoluble.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Copper(Ii) Sulfate Soluble Or Insoluble All sulfates are soluble except strontium sulfate and barium sulfate. It is highly soluble in water. The compound melts at 110°c (230°f) with decomposition. Or determine the solubility using the solubility rules. Copper (ii) sulfate can be prepared in the. Enter a chemical formula of a substance to find its solubility using our solubility calculator. 133 rows all carbonates, sulfides,. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Copper(Ii) Sulfate Soluble Or Insoluble Or determine the solubility using the solubility rules. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Copper (ii) sulfate can be prepared in the. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble.. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.researchgate.net
Temperature dependence of the solubility of copper sulfate in water Copper(Ii) Sulfate Soluble Or Insoluble Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. Or determine the solubility using the solubility rules. Another good way is to reduce it with iron or zinc to. Titration must be used if the reactants are soluble. The compound melts at 110°c (230°f) with. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.sciencephoto.com
Hydrated copper (II) sulphate crystals Stock Image C004/7690 Copper(Ii) Sulfate Soluble Or Insoluble 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. Copper (ii) sulfate can be prepared in the. Another good way is to reduce it with iron or zinc to. The compound melts at 110°c (230°f) with decomposition. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. It is highly. Copper(Ii) Sulfate Soluble Or Insoluble.
From mavink.com
Solubility Curve Chart Copper(Ii) Sulfate Soluble Or Insoluble Another good way is to reduce it with iron or zinc to. All sulfates are soluble except strontium sulfate and barium sulfate. Titration must be used if the reactants are soluble. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. The compound melts at 110°c. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.gradegorilla.com
iGCSE Chemistry Solubility Grade Gorilla Copper(Ii) Sulfate Soluble Or Insoluble Enter a chemical formula of a substance to find its solubility using our solubility calculator. The compound melts at 110°c (230°f) with decomposition. It is highly soluble in water. 133 rows all carbonates, sulfides, oxides and hydroxides are insoluble. All sulfates are soluble except strontium sulfate and barium sulfate. Soluble salts can be made by reacting acids with soluble or. Copper(Ii) Sulfate Soluble Or Insoluble.
From chemicaldb.netlify.app
Copper ii sulfate solubility Copper(Ii) Sulfate Soluble Or Insoluble Titration must be used if the reactants are soluble. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic waste. It is highly soluble in water. Another good way is to reduce it with iron or zinc to. Enter a chemical formula of a substance to find. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.youtube.com
DEMONSTRATING USING COPPER(II) SULFATE TO DETECT WATER A REVERSIBLE Copper(Ii) Sulfate Soluble Or Insoluble Or determine the solubility using the solubility rules. Copper (ii) sulfate can be prepared in the. All sulfates are soluble except strontium sulfate and barium sulfate. The compound melts at 110°c (230°f) with decomposition. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Enter a chemical formula. Copper(Ii) Sulfate Soluble Or Insoluble.
From courses.lumenlearning.com
Solubility Chemistry Atoms First Copper(Ii) Sulfate Soluble Or Insoluble Copper (ii) sulfate can be prepared in the. All sulfates are soluble except strontium sulfate and barium sulfate. Copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it as toxic. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.flinnsci.com
Copper(II) Sulfate, Powder, Lab Grade, 500 g Flinn Scientific Copper(Ii) Sulfate Soluble Or Insoluble Titration must be used if the reactants are soluble. Enter a chemical formula of a substance to find its solubility using our solubility calculator. Another good way is to reduce it with iron or zinc to. Copper (ii) sulfate can be prepared in the. Or determine the solubility using the solubility rules. The compound melts at 110°c (230°f) with decomposition.. Copper(Ii) Sulfate Soluble Or Insoluble.
From www.alamy.com
Copper sulphate solution. Solution of copper (II) sulphate (CuSO4, blue Copper(Ii) Sulfate Soluble Or Insoluble Another good way is to reduce it with iron or zinc to. The compound melts at 110°c (230°f) with decomposition. Soluble salts can be made by reacting acids with soluble or insoluble reactants. It is highly soluble in water. Copper sulfate can be disposed of by either precipitating it as the copper(ii) carbonate, which is insoluble, and disposing of it. Copper(Ii) Sulfate Soluble Or Insoluble.