Household Bleach Redox Reaction . Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. This iodine is then titrated with standardised 1.950 mol. 10.0 cm 3 of bleach was made up to. In the two redox reactions in this experiment, the. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household.
from www.worksheetsplanet.com
In the two redox reactions in this experiment, the. This iodine is then titrated with standardised 1.950 mol. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). 10.0 cm 3 of bleach was made up to. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach.
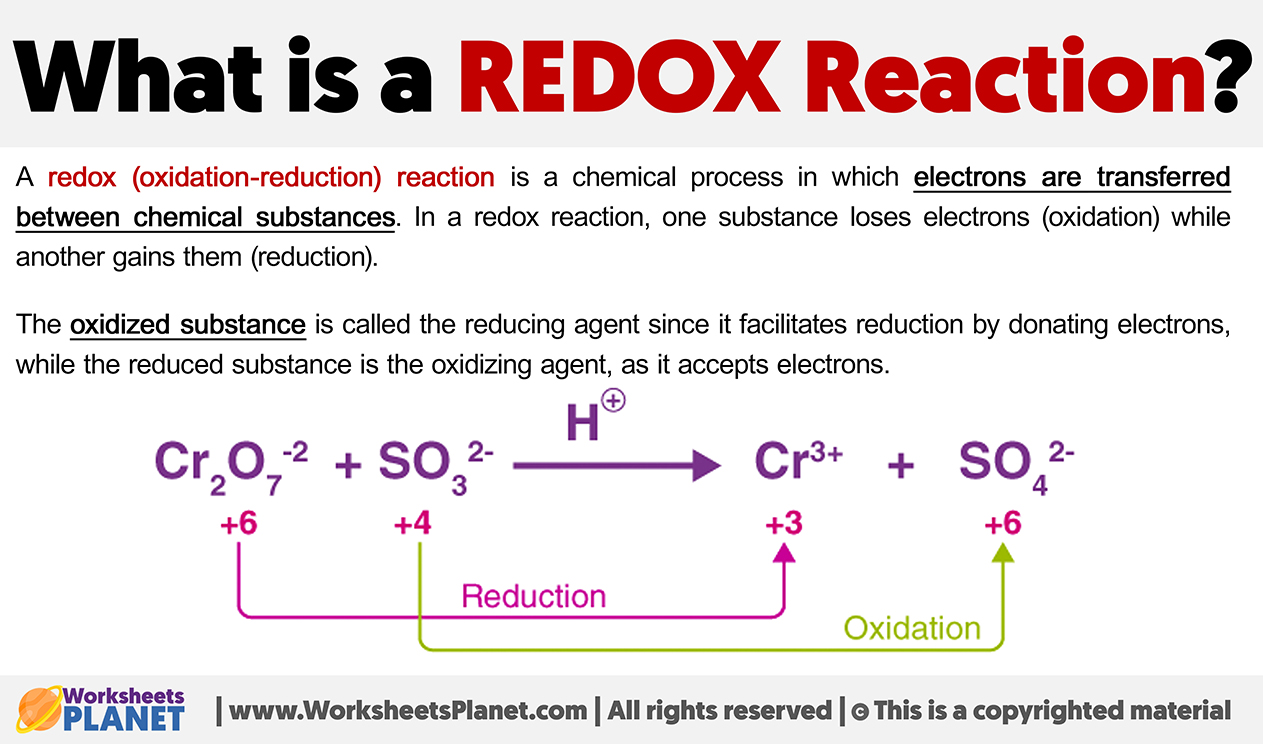
What is a REDOX Reaction?
Household Bleach Redox Reaction Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. 10.0 cm 3 of bleach was made up to. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. This iodine is then titrated with standardised 1.950 mol. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. In the two redox reactions in this experiment, the. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki).
From www.numerade.com
SOLVED 1. Household bleach (NaOCl) is really a solution of sodium Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. This iodine is then titrated with standardised 1.950 mol. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Most likely when you hear. Household Bleach Redox Reaction.
From www.chegg.com
Solved Bleach Oxidation Mechanism Write Out The Complete Household Bleach Redox Reaction Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. In the two redox reactions in this experiment, the. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). 10.0 cm 3 of bleach was made up to. A 20.00 ml sample of household bleach. Household Bleach Redox Reaction.
From www.slideserve.com
PPT OxidationReduction Titrations II Analysis of Bleach PowerPoint Household Bleach Redox Reaction A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. This iodine is then titrated with standardised 1.950 mol. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. Stain. Household Bleach Redox Reaction.
From www.numerade.com
SOLVED This topic is about redox titrations determination of Household Bleach Redox Reaction A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. The two salts that are oxidizing agent's in common household chlorine bleaches. Household Bleach Redox Reaction.
From www.bartleby.com
Answered 4. Balancing an equation for a redox… bartleby Household Bleach Redox Reaction 10.0 cm 3 of bleach was made up to. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. This iodine is then titrated with standardised 1.950 mol. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. A 20.00 ml sample of household bleach is reacted with excess. Household Bleach Redox Reaction.
From www.youtube.com
Making Bleach AS Chemistry YouTube Household Bleach Redox Reaction A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. 10.0 cm 3 of bleach was made up to. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. In the two redox reactions in this experiment, the. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of. Household Bleach Redox Reaction.
From www.slideshare.net
Redox Reactions Household Bleach Redox Reaction Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. 10.0 cm 3 of bleach was made up to. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Household Bleach Redox Reaction.
From www.chegg.com
Solved You will need some bleach to perform a REDOX reaction Household Bleach Redox Reaction In the two redox reactions in this experiment, the. This iodine is then titrated with standardised 1.950 mol. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. The two salts that are oxidizing agent's in common. Household Bleach Redox Reaction.
From www.youtube.com
Lec 14 Redox Reaction Oxidation number of each chlorine in Household Bleach Redox Reaction The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Ordinary household bleach, sodium hypochlorite (naclo), acts on a. Household Bleach Redox Reaction.
From scienceinfo.com
Redox Reactions 4 Types, Significance, Examples Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. In the two redox reactions in this experiment, the. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. 10.0 cm 3 of bleach was made up to. This iodine is then titrated with standardised 1.950 mol. Most. Household Bleach Redox Reaction.
From www.slideserve.com
PPT Booklet to help with Unit 27 PowerPoint Presentation, free Household Bleach Redox Reaction Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). In the two redox reactions in this experiment, the. 10.0. Household Bleach Redox Reaction.
From www.worksheetsplanet.com
What is a REDOX Reaction? Household Bleach Redox Reaction 10.0 cm 3 of bleach was made up to. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). In the two redox reactions in this experiment, the. Stain ‘removal’ is usually decolorizing, or. Household Bleach Redox Reaction.
From slideplayer.com
Redox Reactions Around Us ppt download Household Bleach Redox Reaction Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. In the two redox reactions in this experiment, the. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. Stain. Household Bleach Redox Reaction.
From merinyjoreenmatias.weebly.com
TASK 2 OXIDATION AND REDUCTION PRINCIPLES IN BIOCHEMISTRY Household Bleach Redox Reaction Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. 10.0 cm 3 of bleach was made up to. In the two. Household Bleach Redox Reaction.
From slideplayer.com
Classification and Balancing of Chemical Reactions ppt download Household Bleach Redox Reaction This iodine is then titrated with standardised 1.950 mol. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. In the two redox reactions in this experiment, the. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. 10.0 cm 3 of bleach was made up to. The two. Household Bleach Redox Reaction.
From www.sciencephoto.com
Redox reaction, illustration Stock Image F041/9933 Science Photo Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. In the two redox reactions in this experiment, the. This. Household Bleach Redox Reaction.
From slideplayer.com
Types of Redox Reactions ppt download Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. A 20.00 ml sample of household bleach is reacted with. Household Bleach Redox Reaction.
From brainly.in
explain the applications of redox reaction in bleaching Brainly.in Household Bleach Redox Reaction This iodine is then titrated with standardised 1.950 mol. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). In the two redox reactions in this experiment, the. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. Stain ‘removal’ is usually decolorizing, or. Household Bleach Redox Reaction.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Household Bleach Redox Reaction The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. 10.0 cm 3 of bleach was made up to. This iodine is then titrated with standardised 1.950 mol. Most likely when you hear the. Household Bleach Redox Reaction.
From www.slideserve.com
PPT Redox Titration OF bleach PowerPoint Presentation, free download Household Bleach Redox Reaction Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite. Household Bleach Redox Reaction.
From www.chegg.com
Solved Given the mechanism for bleach's oxidation of Household Bleach Redox Reaction A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. 10.0 cm 3 of bleach was made up to. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. This iodine is then titrated with. Household Bleach Redox Reaction.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID1951818 Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. This iodine is then titrated with standardised 1.950 mol. 10.0 cm 3 of bleach was made up to. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal. Household Bleach Redox Reaction.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation, free download ID6357607 Household Bleach Redox Reaction In the two redox reactions in this experiment, the. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). A 20.00. Household Bleach Redox Reaction.
From www.slideserve.com
PPT “OxidationReduction Reactions” PowerPoint Presentation, free Household Bleach Redox Reaction Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. 10.0 cm 3 of bleach was made up to. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide. Household Bleach Redox Reaction.
From slideplayer.com
Unit 4 Chemical Quantities and Aqueous Reactions ppt download Household Bleach Redox Reaction 10.0 cm 3 of bleach was made up to. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). Most likely when you hear the word ‘bleach,’ you think of clorox® laundry. Household Bleach Redox Reaction.
From slidetodoc.com
Electrochemistry Redox Reactions Day 1 Section 9 1 Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. In the two redox reactions in this experiment, the. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. This iodine is. Household Bleach Redox Reaction.
From www.slideshare.net
Redox Household Bleach Redox Reaction Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. This iodine is then titrated with standardised 1.950 mol. The two salts that are oxidizing. Household Bleach Redox Reaction.
From cartoondealer.com
Chlorine Dioxide ClO2 Molecule. Used In Pulp Bleaching And For Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process. Household Bleach Redox Reaction.
From www.youtube.com
Bleach and Hydrogen Peroxide Reaction + Balanced Equation YouTube Household Bleach Redox Reaction 10.0 cm 3 of bleach was made up to. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. This. Household Bleach Redox Reaction.
From www.slideserve.com
PPT OxidationReduction Titrations II Analysis of Bleach PowerPoint Household Bleach Redox Reaction In the two redox reactions in this experiment, the. 10.0 cm 3 of bleach was made up to. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. Show how redox reactions can be used to determine. Household Bleach Redox Reaction.
From www.scribd.com
Postlab 8 Bleach Redoxproblems PDF Redox Chemical Reactions Household Bleach Redox Reaction Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. The two salts that are oxidizing agent's in common household chlorine bleaches are sodium hypochlorite (naocl) and potassium iodide (ki). A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. 10.0 cm 3 of bleach was made up to.. Household Bleach Redox Reaction.
From sciencenotes.org
Redox Reactions Identify and Balance Oxidation and Reduction Household Bleach Redox Reaction 10.0 cm 3 of bleach was made up to. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. In the two redox reactions in this experiment, the. This iodine is then titrated with standardised 1.950 mol. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household.. Household Bleach Redox Reaction.
From eduinput.com
10 Examples of Redox Reactions Household Bleach Redox Reaction Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. In the two redox reactions in this experiment, the. Ordinary household bleach, sodium hypochlorite (naclo),. Household Bleach Redox Reaction.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Household Bleach Redox Reaction Show how redox reactions can be used to determine the amount of hypochlorite in household bleach. Stain ‘removal’ is usually decolorizing, or bleaching, not complete removal of the stain molecules. Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. This iodine is then titrated with standardised 1.950 mol. In the two redox reactions in. Household Bleach Redox Reaction.
From pubs.acs.org
Reactivity of Household Oxygen Bleaches A Stepwise Laboratory Exercise Household Bleach Redox Reaction Most likely when you hear the word ‘bleach,’ you think of clorox® laundry and household. This iodine is then titrated with standardised 1.950 mol. Ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation reduction, or. A 20.00 ml sample of household bleach is reacted with excess iodide ions to produce iodine. 10.0 cm. Household Bleach Redox Reaction.