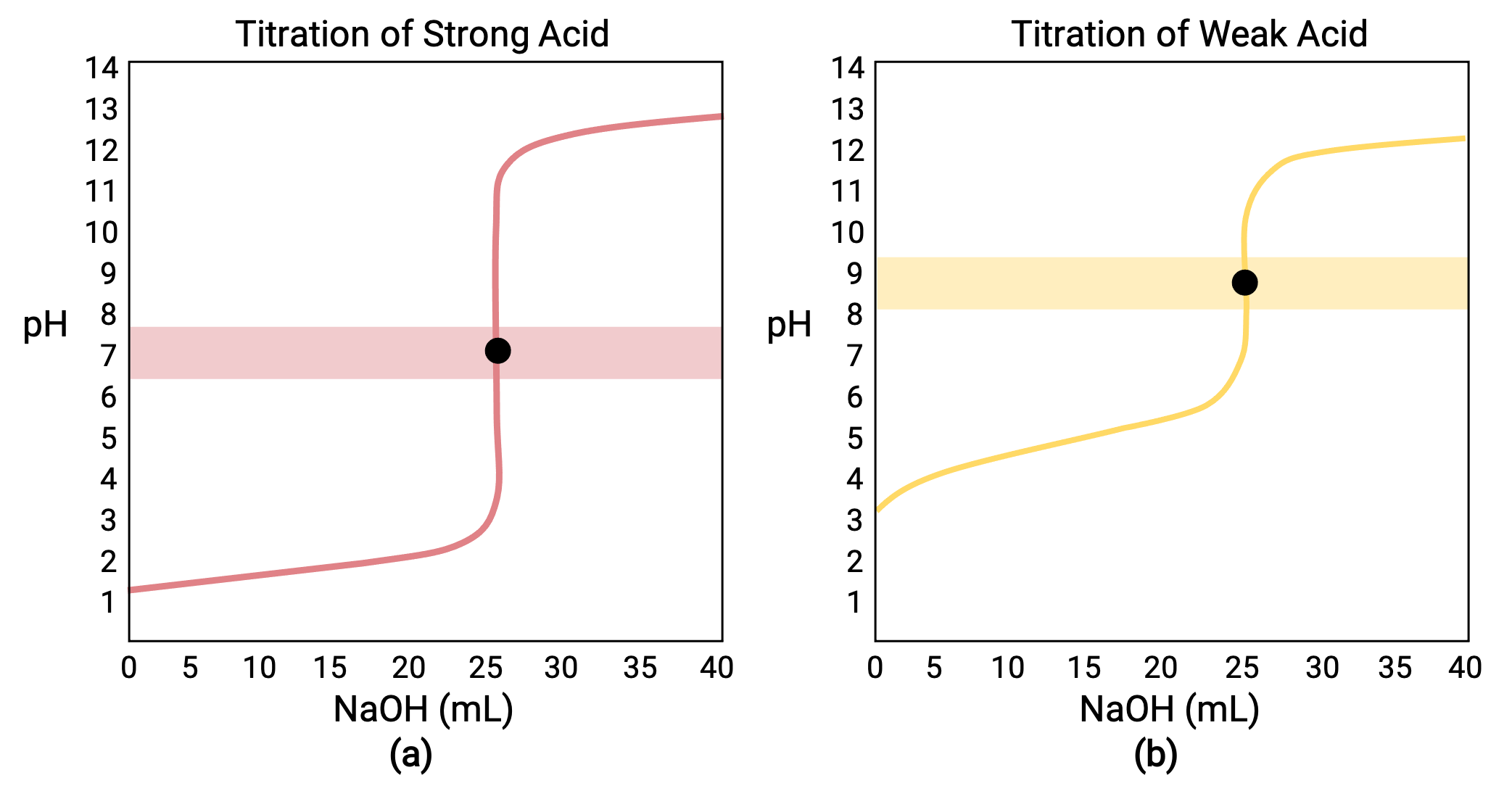
Titration Jump . Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The equivalence point of a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. The successive k a 's must differ by. These plots can be constructed by plotting the ph as a function of. Both equivalence points are visible. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one.
from app.jove.com
The equivalence point of a titration. These plots can be constructed by plotting the ph as a function of. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The successive k a 's must differ by. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. Both equivalence points are visible. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.
AcidBase/ pH Titration Curves and Equivalence Points Concept
Titration Jump The successive k a 's must differ by. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. Both equivalence points are visible. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. The equivalence point of a titration. These plots can be constructed by plotting the ph as a function of. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. The successive k a 's must differ by.
From www.aiophotoz.com
What Is A Titration Images and Photos finder Titration Jump In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. Depending on the. Titration Jump.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Titration Jump Both equivalence points are visible. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The equivalence point of a titration. These plots can be constructed by plotting the ph as a function of. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength. Titration Jump.
From www.numerade.com
SOLVED Calculate the jump in the titration curve when a solution of Titration Jump A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Both equivalence points are visible. The actual magnitude of the jump in ph, and the ph range which it covers. Titration Jump.
From www.researchgate.net
Potentiometric titration curve of lignosulfonates. ERC, Δ pH/ΔV; EP1 Titration Jump The successive k a 's must differ by. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator. Titration Jump.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Jump In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. These plots can. Titration Jump.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Jump In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on. Titration Jump.
From www.vrogue.co
Titration 4 Redox Titration End Point Titration Scree vrogue.co Titration Jump Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: A plot showing the ph of the solution as a function of the. Titration Jump.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Jump Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. A plot showing the ph of the solution as a function of the quantity. Titration Jump.
From mavink.com
Acid Base Titration Equation Titration Jump Both equivalence points are visible. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. Depending on the strength of the acid in the specific solvent, this may. Titration Jump.
From www.vrogue.co
Types Of Titration Acid Base Titrations Redox Amp Com vrogue.co Titration Jump A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. The equivalence point of a titration. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. The successive k a. Titration Jump.
From mavink.com
Titration Reaction Titration Jump Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The equivalence point of a titration. The successive k a 's must differ. Titration Jump.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME Titration Jump Both equivalence points are visible. The successive k a 's must differ by. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration. Titration Jump.
From mungfali.com
Acid Base Titration Procedure Titration Jump The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. The equivalence point of a titration. Both equivalence points are visible. Depending on the strength of the acid in the specific solvent, this. Titration Jump.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Jump These plots can be constructed by plotting the ph as a function of. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. The successive k a 's must differ by. Both equivalence points are visible. In general, there are two requirements for. Titration Jump.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Jump The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve.. Titration Jump.
From saylordotorg.github.io
AcidBase Titrations Titration Jump The successive k a 's must differ by. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. Both equivalence points are visible. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A plot showing the ph of the solution as a function. Titration Jump.
From chem.libretexts.org
14.10 Titration Curves Chemistry LibreTexts Titration Jump The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. The equivalence point of a titration. These plots can be constructed by plotting the ph as a function of. A plot showing the. Titration Jump.
From scienceready.com.au
Titration pH Curves HSC Chemistry Science Ready Titration Jump These plots can be constructed by plotting the ph as a function of. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and. Titration Jump.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Jump Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. The equivalence point of a titration. These plots can be constructed by plotting the. Titration Jump.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Jump The equivalence point of a titration. The successive k a 's must differ by. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility.. Titration Jump.
From www.vrogue.co
Acid Base Titration Purpose vrogue.co Titration Jump The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In general, there are two requirements. Titration Jump.
From www.savemyexams.co.uk
AcidAlkali Titrations (2.6.3) Edexcel IGCSE Chemistry Revision Notes Titration Jump In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a. Titration Jump.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Jump In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. Precipitation titration is one of the four important titration methods in analytical chemistry,. Titration Jump.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Jump Both equivalence points are visible. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. The actual magnitude of the jump in ph, and the ph range. Titration Jump.
From www.researchgate.net
(a) Integral and (b) differential pHmetric titration curves of an Titration Jump These plots can be constructed by plotting the ph as a function of. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. The successive k a 's must differ by. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based. Titration Jump.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration Jump A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The equivalence point of a titration. Precipitation titration is one of the four important titration methods in. Titration Jump.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Jump A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. In general, there are two requirements for a clearly discernible jump in the ph to occur in a. Titration Jump.
From www.animalia-life.club
Titration Curve Amino Acid Titration Jump Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. Precipitation titration is one of the four important titration methods in analytical chemistry, which is based on the solubility. In general, there are two requirements for a clearly discernible jump in the ph. Titration Jump.
From saylordotorg.github.io
AcidBase Titrations Titration Jump The equivalence point of a titration. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: These plots can be constructed by plotting the ph as a function of. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Precipitation titration is one. Titration Jump.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Jump The successive k a 's must differ by. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so. Titration Jump.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Jump The actual magnitude of the jump in ph, and the ph range which it covers depend on the strength of both the acid and the base involved, and so the choice of indicator can vary from one. The equivalence point of a titration. In general, there are two requirements for a clearly discernible jump in the ph to occur in. Titration Jump.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Jump In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a. Titration Jump.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Titration Jump The equivalence point of a titration. The successive k a 's must differ by. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. A plot showing the ph of the solution as a function of the quantity of base added is known. Titration Jump.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A Titration Jump A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration. In general, there are two requirements for a clearly discernible jump in the ph to occur. Titration Jump.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Jump Both equivalence points are visible. The successive k a 's must differ by. These plots can be constructed by plotting the ph as a function of. The equivalence point of a titration. A plot showing the ph of the solution as a function of the quantity of base added is known as a titration curve. A typical titration curve of. Titration Jump.