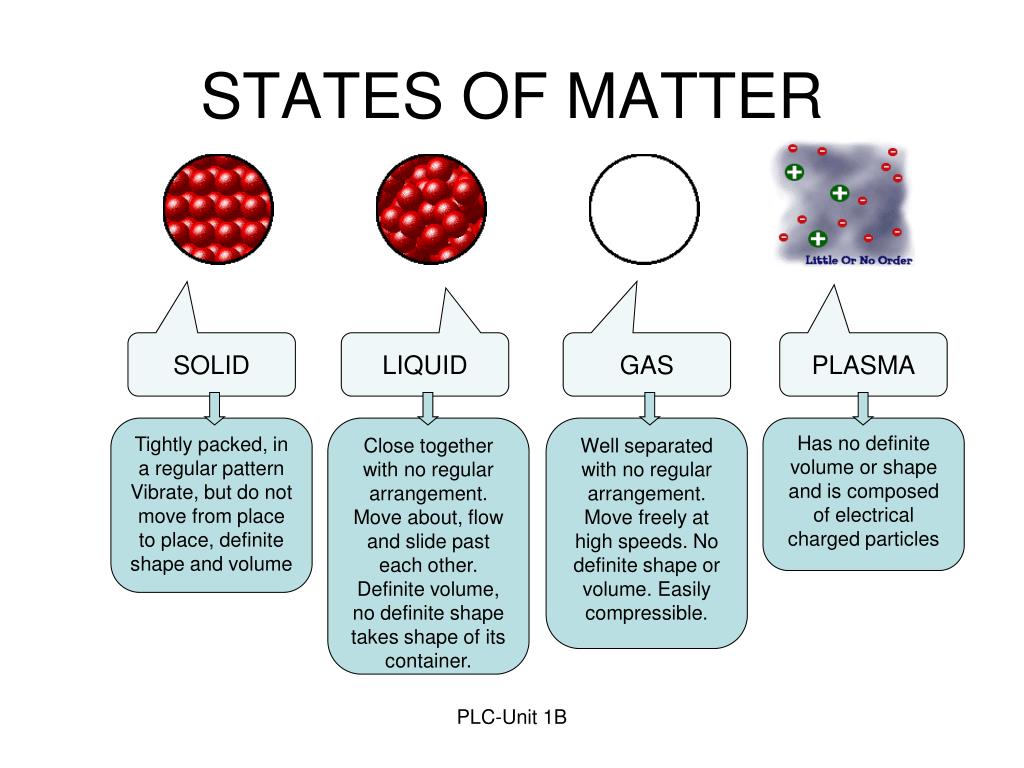
Solid Liquid Kinetic Theory . You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Particles in solids, liquids and gases have different amounts of energy. Kinetic theory is the atomistic description of gases as well as liquids and solids. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Slowly moving molecules are attracted to each other easily and results in condensed phases: The theory helps explain observable properties and behaviors of solids, liquids, and gases. Study how it interacts with gas laws, and view examples. Kinetic theory models the properties of matter in terms of continuous random motion of atoms and molecules. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The particles are arranged differently and move in different ways. Describe the behavior of an ideal gas.
from www.slideserve.com
The theory helps explain observable properties and behaviors of solids, liquids, and gases. Slowly moving molecules are attracted to each other easily and results in condensed phases: Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Study how it interacts with gas laws, and view examples. Kinetic theory models the properties of matter in terms of continuous random motion of atoms and molecules. The particles are arranged differently and move in different ways. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Particles in solids, liquids and gases have different amounts of energy. Describe the behavior of an ideal gas.
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint
Solid Liquid Kinetic Theory If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The particles are arranged differently and move in different ways. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Describe the behavior of an ideal gas. Slowly moving molecules are attracted to each other easily and results in condensed phases: Kinetic theory is the atomistic description of gases as well as liquids and solids. Particles in solids, liquids and gases have different amounts of energy. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Study how it interacts with gas laws, and view examples. Kinetic theory models the properties of matter in terms of continuous random motion of atoms and molecules. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Study how it interacts with gas laws, and view examples. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to. Solid Liquid Kinetic Theory.
From www.youtube.com
molecular theory of Liquid and Solid YouTube Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. Kinetic theory is the atomistic description of gases as well as liquids and solids. Slowly moving molecules are attracted to each other easily and results in condensed phases: The theory helps explain observable properties and behaviors of solids, liquids, and gases. Study how it interacts with gas laws, and. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Particle Theory PowerPoint Presentation, free download Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Describe the behavior of an ideal gas. Study how it interacts with gas laws, and view examples. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Solid Liquid Kinetic Theory Slowly moving molecules are attracted to each other easily and results in condensed phases: Particles in solids, liquids and gases have different amounts of energy. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. The theory helps explain observable properties and behaviors of solids, liquids, and. Solid Liquid Kinetic Theory.
From www.youtube.com
theory of Solids, Liquids & Gases IGCSE Chemistry Learn Solid Liquid Kinetic Theory If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Particles in solids, liquids and gases have different amounts of energy. You can explain the differences in the physical properties of solids, liquids and gases by referring to the. Solid Liquid Kinetic Theory.
From www.slideshare.net
Theory AQA 2012 P1 Solid Liquid Kinetic Theory Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Describe the behavior of an ideal gas. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Study how it interacts with gas laws, and view examples. Particles in solids, liquids and gases have different amounts of energy. Kinetic theory. Solid Liquid Kinetic Theory.
From thinktivity.wixsite.com
Theory of Matter Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. Slowly moving molecules are attracted to each other easily and results in condensed phases: The theory helps explain observable properties and behaviors of solids, liquids, and gases. Describe the behavior of an ideal gas. The particles are arranged differently and move in different ways. If energy is supplied by. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Particle Theory PowerPoint Presentation, free download Solid Liquid Kinetic Theory The theory helps explain observable properties and behaviors of solids, liquids, and gases. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Slowly moving molecules are attracted to each other easily and results in condensed phases: Kinetic theory is the atomistic description of gases as well as liquids and solids. Kinetic theory. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT I. Physical Properties PowerPoint Presentation, free download Solid Liquid Kinetic Theory The theory helps explain observable properties and behaviors of solids, liquids, and gases. Particles in solids, liquids and gases have different amounts of energy. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Kinetic theory models the properties of matter in terms of continuous random motion. Solid Liquid Kinetic Theory.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases Solid Liquid Kinetic Theory Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Kinetic theory is the atomistic description of gases as well as liquids and solids. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Slowly moving molecules are attracted to each other easily and results in condensed phases: Describe the. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Solid Liquid Kinetic Theory Describe the behavior of an ideal gas. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The particles are arranged differently and move in different ways. Study how it. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Particle Theory PowerPoint Presentation, free download Solid Liquid Kinetic Theory You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Describe the behavior of an ideal gas. Particles in solids, liquids and gases have different amounts of energy. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Molecular Theory of Solids and Liquids PowerPoint Solid Liquid Kinetic Theory The particles are arranged differently and move in different ways. Describe the behavior of an ideal gas. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Kinetic theory is the atomistic description of gases as well as liquids and solids. Particles in solids, liquids and gases have different amounts of energy. If energy is supplied by. Solid Liquid Kinetic Theory.
From www.sciencelearn.org.nz
model of matter — Science Learning Hub Solid Liquid Kinetic Theory If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Slowly moving molecules are attracted to each other easily and results in condensed phases: The theory helps explain observable properties and behaviors of solids, liquids, and gases. Kinetic theory. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Particle Theory Model of Matter) PowerPoint Solid Liquid Kinetic Theory You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Particles in solids, liquids and gases have different amounts of energy. The theory helps explain observable properties and behaviors of. Solid Liquid Kinetic Theory.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Liquid Kinetic Theory The particles are arranged differently and move in different ways. Study how it interacts with gas laws, and view examples. The theory helps explain observable properties and behaviors of solids, liquids, and gases. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid. Solid Liquid Kinetic Theory.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The particles are arranged differently and move in different ways. Kinetic theory models the properties of matter in terms of continuous random motion of atoms and molecules. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The particles are arranged differently and move in different ways. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to. Solid Liquid Kinetic Theory.
From www.shutterstock.com
Theory Matter Infographic Diagram Showing Stock Illustration Solid Liquid Kinetic Theory Kinetic theory is the atomistic description of gases as well as liquids and solids. Describe the behavior of an ideal gas. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Kinetic theory models the properties of matter in. Solid Liquid Kinetic Theory.
From www.vecteezy.com
theory, Explanation of States of Matter, Changing states of Solid Liquid Kinetic Theory If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The particles are arranged differently and move in different ways. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Slowly moving molecules are attracted to. Solid Liquid Kinetic Theory.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Particles & Equations Solid Liquid Kinetic Theory Slowly moving molecules are attracted to each other easily and results in condensed phases: If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Study how it interacts with gas laws, and view examples. Particles in solids, liquids and. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free Solid Liquid Kinetic Theory You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Kinetic theory is the atomistic description of gases as well as liquids and solids. Slowly moving molecules are attracted to each other easily and results in condensed phases: Study how it interacts with gas laws, and view. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces PowerPoint Solid Liquid Kinetic Theory The theory helps explain observable properties and behaviors of solids, liquids, and gases. Particles in solids, liquids and gases have different amounts of energy. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Kinetic theory models the properties. Solid Liquid Kinetic Theory.
From studyrocket.co.uk
The Particle Model GCSE Chemistry Science) OCR Revision Solid Liquid Kinetic Theory Kinetic theory models the properties of matter in terms of continuous random motion of atoms and molecules. Slowly moving molecules are attracted to each other easily and results in condensed phases: Study how it interacts with gas laws, and view examples. Kinetic theory is the atomistic description of gases as well as liquids and solids. If energy is supplied by. Solid Liquid Kinetic Theory.
From www.britannica.com
phase Definition & Facts Britannica Solid Liquid Kinetic Theory Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The particles are arranged differently and move in different ways. Particles in solids, liquids and gases have different amounts of energy. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to. Solid Liquid Kinetic Theory.
From x-engineer.org
The states (phases) of matter (aggregation) Solid Liquid Kinetic Theory Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The particles are arranged differently and move in different ways. Particles in solids, liquids and gases have different amounts of energy. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT The Theory of Matter PowerPoint Presentation, free Solid Liquid Kinetic Theory Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Kinetic theory models the properties of matter in terms of continuous random. Solid Liquid Kinetic Theory.
From studylib.net
The Molecular Theory of Liquids & Solids Solid Liquid Kinetic Theory Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Slowly moving molecules are attracted to each other easily and results in condensed phases: Kinetic theory models the properties of matter in terms of continuous random motion of atoms and molecules. Particles in solids, liquids and gases have different amounts of energy. If. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Solid Liquid Kinetic Theory Slowly moving molecules are attracted to each other easily and results in condensed phases: The theory helps explain observable properties and behaviors of solids, liquids, and gases. You can explain the differences in the physical properties of solids, liquids and gases by referring to the arrangement and motion of particles. Study how it interacts with gas laws, and view examples.. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID1882261 Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The theory helps explain observable properties and behaviors of solids, liquids, and gases. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Kinetic theory models the properties. Solid Liquid Kinetic Theory.
From slidetodoc.com
CHAPTER 1 Particle Theory 2013 Marshall Cavendish Solid Liquid Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. Describe the behavior of an ideal gas. Kinetic theory is the atomistic description of gases as well as. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID5445725 Solid Liquid Kinetic Theory Kinetic theory is the atomistic description of gases as well as liquids and solids. If energy is supplied by heating a solid, the heat energy causes stronger vibrations until the particles eventually have enough energy to break away from the solid arrangement to form a. The theory helps explain observable properties and behaviors of solids, liquids, and gases. You can. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT Chapter 6 The States of Matter PowerPoint Presentation, free Solid Liquid Kinetic Theory Slowly moving molecules are attracted to each other easily and results in condensed phases: Particles in solids, liquids and gases have different amounts of energy. The particles are arranged differently and move in different ways. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Study how it interacts with gas laws, and. Solid Liquid Kinetic Theory.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation Solid Liquid Kinetic Theory Kinetic theory is the atomistic description of gases as well as liquids and solids. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Particles in solids, liquids and gases have different amounts of energy. The particles are arranged differently and move in different ways. Slowly moving molecules are attracted to each other easily and results in. Solid Liquid Kinetic Theory.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid Liquid Kinetic Theory Study how it interacts with gas laws, and view examples. Particles in solids, liquids and gases have different amounts of energy. The theory helps explain observable properties and behaviors of solids, liquids, and gases. Slowly moving molecules are attracted to each other easily and results in condensed phases: Kinetic theory models the properties of matter in terms of continuous random. Solid Liquid Kinetic Theory.