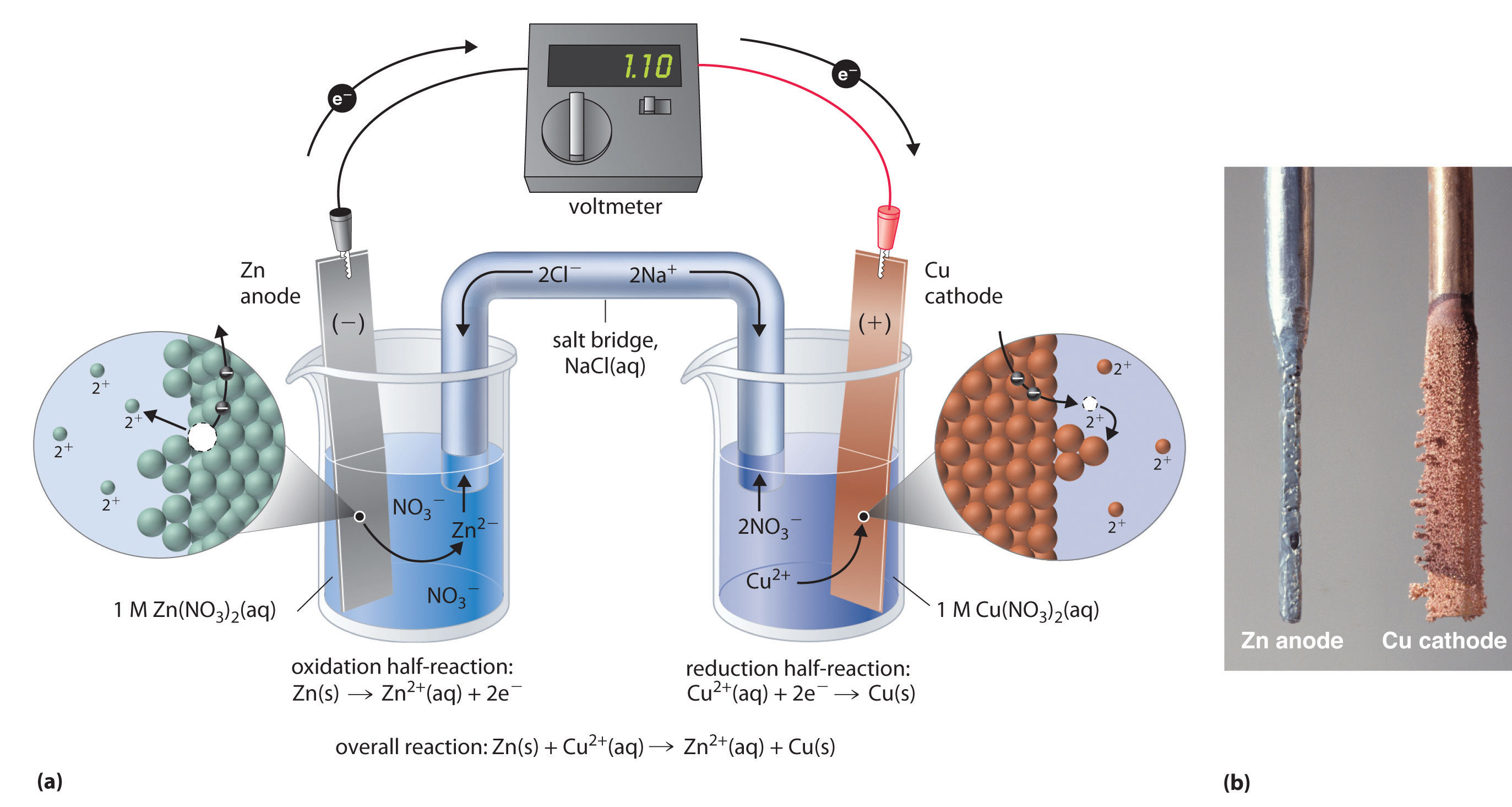
Zinc Charge Reaction . When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): This is one example of what is sometimes. Metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. Battery charge and discharge through these chemical reactions. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Charges predict whether an atom bonds with another atom. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. Metallic zinc reacts with weak acids very slowly. This is a table of the most common charges for atoms of the chemical elements. When heated, the two powders react explosively to form zinc. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. Sulfur has a strong affinity for zinc. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half.
from lessonschoolframboises.z14.web.core.windows.net
To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. This is a table of the most common charges for atoms of the chemical elements. Metallic zinc reacts with weak acids very slowly. When heated, the two powders react explosively to form zinc. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Charges predict whether an atom bonds with another atom. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. When heated, the two powders react explosively to form zinc.
Reaction Of Zinc With Oxygen
Zinc Charge Reaction Metallic zinc reacts with weak acids very slowly. Metallic zinc reacts with weak acids very slowly. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. This is a table of the most common charges for atoms of the chemical elements. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Sulfur has a strong affinity for zinc. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): When heated, the two powders react explosively to form zinc. Sulfur has a strong affinity for zinc. Charges predict whether an atom bonds with another atom. Battery charge and discharge through these chemical reactions. When heated, the two powders react explosively to form zinc. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Metallic zinc reacts with weak acids very slowly.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc Charge Reaction When heated, the two powders react explosively to form zinc. Sulfur has a strong affinity for zinc. When heated, the two powders react explosively to form zinc. The half reaction for the oxidation reaction, omitting phase labels, is as follows: To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. According to whether the. Zinc Charge Reaction.
From chemistryfromscratch.org
V3.9 Zinc Charge Reaction The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. Metallic zinc reacts with weak acids very slowly. To understand oxidation and reduction, let’s look at a chemical reaction between. Zinc Charge Reaction.
From www.youtube.com
How to Balance Zn + O2 = ZnO (Zinc + Oxygen gas) YouTube Zinc Charge Reaction Metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. This is one example of what is sometimes. Charges predict whether an atom bonds with another atom. When heated, the two powders react explosively to form zinc. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. According to whether. Zinc Charge Reaction.
From www.researchgate.net
Rechargeable zincair battery tests. (a) Chargedischarge polarization Zinc Charge Reaction The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. Charges predict whether an atom bonds with another atom. This is a table of the most common charges for atoms of the chemical elements. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. This is one example of what. Zinc Charge Reaction.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Charge Reaction Sulfur has a strong affinity for zinc. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Battery charge and discharge through these chemical reactions. Metallic zinc reacts with. Zinc Charge Reaction.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol Zinc Charge Reaction When heated, the two powders react explosively to form zinc. Battery charge and discharge through these chemical reactions. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. When heated, the two powders react explosively to form zinc. Charges predict whether an atom bonds with another atom. The half. Zinc Charge Reaction.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Charge Reaction When heated, the two powders react explosively to form zinc. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. The half reaction for the oxidation reaction, omitting phase labels, is as follows: \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. This is a table of the most. Zinc Charge Reaction.
From www.sliderbase.com
Ionic Compound Nomenclature Presentation Chemistry Zinc Charge Reaction Sulfur has a strong affinity for zinc. When heated, the two powders react explosively to form zinc. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. Metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. In the above reaction zinc (zn) first gives up. Zinc Charge Reaction.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Charge Reaction Metallic zinc reacts with weak acids very slowly. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. Charges predict whether an atom bonds with another atom. Metallic zinc reacts with weak acids very slowly. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. According to whether the cathode. Zinc Charge Reaction.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Charge Reaction This is one example of what is sometimes. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Sulfur has a strong affinity for zinc. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode. Zinc Charge Reaction.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Charge Reaction According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): Sulfur has a strong affinity for zinc. Charges predict whether an atom bonds with another atom. To understand oxidation. Zinc Charge Reaction.
From www.britannica.com
Zinc Properties, Uses, & Facts Britannica Zinc Charge Reaction Battery charge and discharge through these chemical reactions. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. When heated, the two powders react explosively to form zinc. Sulfur has a strong affinity for zinc. Sulfur has a strong affinity for zinc. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. This is one example. Zinc Charge Reaction.
From www.science.org
A rechargeable zincair battery based on zinc peroxide chemistry Science Zinc Charge Reaction When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Sulfur has a strong affinity for zinc. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine.. Zinc Charge Reaction.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Zinc Charge Reaction This is a table of the most common charges for atoms of the chemical elements. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. This is. Zinc Charge Reaction.
From www.slideserve.com
PPT Corrosion of Metals PowerPoint Presentation ID2978412 Zinc Charge Reaction According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. This is one example of what is sometimes. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Battery charge and discharge through these chemical reactions. To understand oxidation and reduction, let’s look at a chemical reaction between. Zinc Charge Reaction.
From www.researchgate.net
Discharge performance of zincair battery in 1 M KOH Download Zinc Charge Reaction When heated, the two powders react explosively to form zinc. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Sulfur has a strong affinity for zinc. This is one example of what is sometimes. Metallic zinc reacts with. Zinc Charge Reaction.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Zinc Charge Reaction Battery charge and discharge through these chemical reactions. The half reaction for the oxidation reaction, omitting phase labels, is as follows: To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. When heated, the two. Zinc Charge Reaction.
From pubs.rsc.org
In operando monitoring of the state of charge and species distribution Zinc Charge Reaction Charges predict whether an atom bonds with another atom. This is one example of what is sometimes. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. The half reaction for the oxidation reaction,. Zinc Charge Reaction.
From lessonschoolframboises.z14.web.core.windows.net
Reaction Of Zinc With Oxygen Zinc Charge Reaction Metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. This is one example of what is sometimes. This is a table of the most common charges for atoms of the chemical elements. Sulfur has a strong affinity for zinc. When heated, the two powders react explosively to form zinc. When heated, the two powders. Zinc Charge Reaction.
From www.science.org
A rechargeable zincair battery based on zinc peroxide chemistry Science Zinc Charge Reaction When heated, the two powders react explosively to form zinc. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. Metallic zinc reacts with weak acids very slowly. This is a table of. Zinc Charge Reaction.
From edurev.in
The reaction of zinc with dilute and concentrated nitric acid Zinc Charge Reaction Charges predict whether an atom bonds with another atom. Metallic zinc reacts with weak acids very slowly. When heated, the two powders react explosively to form zinc. The half reaction for the oxidation reaction, omitting phase labels, is as follows: To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. \[\ce{zn → zn^{2+} +. Zinc Charge Reaction.
From elchoroukhost.net
Zinc Periodic Table Protons Neutrons Electrons Elcho Table Zinc Charge Reaction Charges predict whether an atom bonds with another atom. This is a table of the most common charges for atoms of the chemical elements. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. This is one example of what is sometimes. To understand oxidation and reduction, let’s look at a. Zinc Charge Reaction.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Charge Reaction Charges predict whether an atom bonds with another atom. Metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus hydrochloric acid): In the above reaction zinc (zn) first gives up two electrons and becomes positive. Zinc Charge Reaction.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Charge Reaction The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. The half reaction for the oxidation reaction, omitting phase labels, is as follows: According to. Zinc Charge Reaction.
From www.youtube.com
Ionic Charge for Zinc (Zn) YouTube Zinc Charge Reaction Sulfur has a strong affinity for zinc. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Metallic zinc reacts with weak acids very slowly. According to. Zinc Charge Reaction.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Charge Reaction \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. This is a table of the most common charges for atoms of the chemical elements. Battery charge and discharge through these chemical reactions. In the above reaction zinc (zn) first gives up. Zinc Charge Reaction.
From www.researchgate.net
(a) Open circuit potential of Zinc air batteries of NiCoCr LDH/NrGO Zinc Charge Reaction When heated, the two powders react explosively to form zinc. This is one example of what is sometimes. When heated, the two powders react explosively to form zinc. Metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. Charges predict whether an atom bonds with another. Zinc Charge Reaction.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Charge Reaction The half reaction for the oxidation reaction, omitting phase labels, is as follows: Sulfur has a strong affinity for zinc. When heated, the two powders react explosively to form zinc. Battery charge and discharge through these chemical reactions. When heated, the two powders react explosively to form zinc. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. When zinc metal. Zinc Charge Reaction.
From melscience.com
La réaction entre l’acide chlorhydrique et le zinc MEL Chemistry Zinc Charge Reaction This is one example of what is sometimes. Sulfur has a strong affinity for zinc. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. Metallic zinc reacts with weak acids very slowly. This. Zinc Charge Reaction.
From sciencetrends.com
What Is The Ionic Charge Of Zinc (Zn)? Science Trends Zinc Charge Reaction Sulfur has a strong affinity for zinc. When heated, the two powders react explosively to form zinc. Metallic zinc reacts with weak acids very slowly. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. When zinc metal is submerged into a quantity of aqueous hcl, the following reaction occurs (figure 5.4 zinc metal plus. Zinc Charge Reaction.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Zinc Charge Reaction When heated, the two powders react explosively to form zinc. Metallic zinc reacts with weak acids very slowly. This is one example of what is sometimes. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Battery charge and. Zinc Charge Reaction.
From www.freepik.com
Free Vector A zinc atom diagram Zinc Charge Reaction Sulfur has a strong affinity for zinc. When heated, the two powders react explosively to form zinc. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this half. Charges predict whether an atom bonds with another atom. The half reaction for the oxidation reaction, omitting phase labels, is as follows: This is a table of the most common charges for atoms of. Zinc Charge Reaction.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Zinc Charge Reaction According to whether the cathode undergoes a phase change during the charging and discharging, the cathode reaction involving zinc. In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Sulfur has a strong affinity for zinc. Battery charge and discharge through these chemical reactions. The reason the electrons leave in the first place (why the. Zinc Charge Reaction.
From www.researchgate.net
(a) Chargedischarge curves of a zincair battery, with air electrodes Zinc Charge Reaction In the above reaction zinc (zn) first gives up two electrons and becomes positive ions. Metallic zinc reacts with weak acids very slowly. Charges predict whether an atom bonds with another atom. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. When heated, the two powders react explosively to form zinc. This is. Zinc Charge Reaction.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Charge Reaction When heated, the two powders react explosively to form zinc. Metallic zinc reacts with weak acids very slowly. The reason the electrons leave in the first place (why the oxidation reaction above occurs) is that it is more energetically. To understand oxidation and reduction, let’s look at a chemical reaction between zinc metal and chlorine. Metallic zinc reacts with weak. Zinc Charge Reaction.